Key Points
ECM is associated with an early marked increase in plasma VWF levels and accumulation of UL-VWF multimers.
Following P berghei infection, VWF−/− mice survive significantly longer compared with WT controls.
Abstract
Plasmodium falciparum malaria infection is associated with an early marked increase in plasma von Willebrand factor (VWF) levels, together with a pathological accumulation of hyperreactive ultra-large VWF (UL-VWF) multimers. Given the established critical role of platelets in malaria pathogenesis, these increases in plasma VWF raise the intriguing possibility that VWF may play a direct role in modulating malaria pathogenesis. To address this hypothesis, we used an established murine model of experimental cerebral malaria (ECM), in which wild-type (WT) C57BL/6J mice were infected with Plasmodium berghei ANKA. In keeping with findings in children with P falciparum malaria, acute endothelial cell activation was an early and consistent feature in the murine model of cerebral malaria (CM), resulting in significantly increased plasma VWF levels. Despite the fact that murine plasma ADAMTS13 levels were not significantly reduced, pathological UL-VWF multimers were also observed in murine plasma following P berghei infection. To determine whether VWF plays a role in modulating the pathogenesis of CM in vivo, we further investigated P berghei infection in VWF−/− C57BL/6J mice. Clinical ECM progression was delayed, and overall survival was significantly prolonged in VWF−/− mice compared with WT controls. Despite this protection against ECM, no significant differences in platelet counts or blood parasitemia levels were observed between VWF−/− and WT mice. Interestingly, however, the degree of ECM-associated enhanced blood–brain barrier permeability was significantly attenuated in VWF−/− mice compared with WT controls. Given the significant morbidity and mortality associated with CM, these novel data may have direct translational significance.
Introduction
Plasmodium falciparum malaria remains a major cause of morbidity and mortality among children in sub-Saharan Africa.1-3 Although the biological mechanisms involved in the pathophysiology of severe P falciparum malaria remain poorly understood, previous studies have demonstrated that sequestration of P falciparum–infected erythrocytes (IEs) within the microvasculature of the brain is important in the development of cerebral malaria (CM).4,5 This sequestration involves adhesion of IE to host vascular endothelial cell (EC) surfaces6-8 and is mediated by a variety of specific EC adhesion molecules including CD36, intercellular adhesion molecule-1, and thrombospondin.9 Moreover, recent studies have also demonstrated that the endothelial protein C receptor also plays an important role in modulating the sequestration of IE.10 In addition to IE, sequestration of leukocytes and platelets within the cerebral microvasculature has also been reported in postmortem samples from CM patients.11,12
Von Willebrand factor (VWF) is a large sialoglycoprotein synthesized within EC and megakarocytes.13 VWF circulates in normal plasma as a series of heterogeneous multimers and plays a critical role in primary hemostasis by mediating platelet adhesion to exposed collagen at sites of vascular injury.14 The multimeric composition of plasma VWF plays a key role in determining its functional activity. In particular, high-molecular-weight multimers of VWF demonstrate enhanced binding affinities for both collagen and platelets and are therefore more efficient in mediating platelet recruitment.14 Following synthesis within EC, VWF is either constitutively secreted into the plasma or else stored within specific intracellular organelles known as Weibel-Palade (WP) bodies.15 This WP-stored VWF is enriched in high-molecular-weight multimers and is actively secreted following EC activation.
In previous studies, we and others have demonstrated that plasma levels of VWF antigen (VWF:Ag) and VWF propeptide (VWF:pp) are markedly elevated in African children with severe P falciparum malaria.16-20 This observation has subsequently been confirmed in other studies that enrolled both children and adult patients with either P falciparum or Plasmodium vivax infections, from a number of different geographical regions.21,22 Interestingly, a study of healthy volunteers infected with P falciparum has also shown that the increase in plasma VWF:Ag and VWF:pp levels is present from a very early stage following the onset of blood-stage infection.23 Collectively, these data demonstrate that marked EC activation, with consequent VWF secretion from WP bodies, constitutes an early hallmark of P falciparum malaria infection.
In addition to the marked increase in plasma VWF:Ag observed in patients with malaria infection, severe P falciparum infection is also associated with a pathological accumulation of ultra-large VWF (UL-VWF) multimers in the plasma of patients.17,21 The molecular mechanism(s) responsible for the presence of these UL-VWF multimers remains unclear. Importantly, however, only modest reductions in plasma ADAMTS13 levels have been reported in malaria-infected patients.17,21,22 Nevertheless, the combination of markedly elevated VWF:Ag levels and hyperreactive UL-VWF multimers in the plasma raises the intriguing possibility that VWF may play a novel role in the pathogenesis of P falciparum malaria. This hypothesis is supported by several recent independent observations. First, plasma VWF:pp levels in children with severe malaria have been shown to correlate with other established biochemical markers of malaria severity, including plasma lactate levels.16 In addition, plasma VWF levels also correlate inversely with platelet count and with overall clinical outcome.16,24 Second, de Mast et al have demonstrated that in patients with P falciparum infection, a significant proportion of plasma VWF is circulating in an active confirmation that promotes platelet glycoprotein Ib binding.24 This observation is important because platelet adhesion and accumulation have been implicated in facilitating the adhesion of P falciparum IE to activated EC.25-27 Finally, in a shear-based assay, we have recently shown that platelet-decorated UL-VWF strings on the surface of activated EC can also recruit trophozoite-stage P falciparum IE.28
The early increase in plasma VWF levels and circulating UL-VWF multimers observed following P falciparum infection poses a challenge in defining whether VWF directly contributes to the development of human CM or whether increased VWF levels merely constitute a secondary epiphenomenon associated with EC activation. In this study, we have sought to further investigate the putative role of VWF in malaria pathogenesis in vivo using an established murine model of experimental cerebral malaria (ECM).
Materials and methods
Murine studies
All mouse experiments were performed in compliance with Irish Medicines Board regulations and were reviewed and approved by the Trinity College Dublin BioResource Ethical Committee. VWF−/− mice were initially obtained from Jackson Laboratories (Bar Harbor, ME). These VWF−/− mice were on a C57BL/6J background and have previously been characterized.29,30 Wild-type (WT) C57BL/6J and VWF−/− mice were bred and maintained in-house under standard pathogen-free conditions. All experiments were performed on mice aged 8 to 10 weeks. Blood samples were collected from either tail vein or by cardiac puncture into acid citrate dextrose anticoagulant (85 mM trisodium citrate, 65 mM citric acid, 100 mM glucose) (Sigma-Aldrich, Wicklow, Ireland). Platelet counts were measured using a Sysmex hematology analyzer (KX-21N). To prepare platelet-poor plasma, blood samples were centrifuged at 1500g for 15 minutes at 20°C, aliquoted, and stored at −80°C until use.
P berghei survival studies
Mice were infected by intraperitoneal injection of 2 × 106Plasmodium berghei ANKA. Following inoculation, malaria progression was monitored using a previously validated clinical scoring system to assess ECM.31 In brief, mice were initially reviewed on a daily basis following P berghei infection. From day +5 postinfection, all mice were assessed every 12 hours. Clinical progression of ECM was determined by examining for the appearance of the following signs: hunched posture, ruffled fur, wobbly gait, limb paralysis, convulsions, and coma. Each sign was awarded a score based on clinical severity (0 or 1). Animals with severe ECM (total cumulative scores >4) were euthanized by cervical dislocation according to ethical guidelines, and day of death was deemed to be the following day. Blood P berghei parasitemia levels were monitored by examination of Giemsa-stained (VWR International Inc) thin blood smears obtained from tail vein bleeds.
Determination of plasma VWF:Ag and collagen-binding activity
Plasma VWF:Ag levels were measured using a previously described enzyme-linked immunosorbent assay (ELISA).32,33 In brief, Maxisorp plates (Nunc, Denmark) were coated with rabbit anti-human VWF antibody (Dako, Glostrup, Denmark) in 50 mM carbonate buffer (pH 9.6). Previous studies have demonstrated that these polyclonal anti-human VWF antibodies cross-react with murine VWF and can be used to measure murine plasma VWF:Ag levels.34,35 After blocking with 3% bovine serum albumin (BSA; Sigma-Aldrich) test samples were then added at appropriate dilutions. Bound murine VWF was detected using horseradish peroxidase–conjugated (HRP) rabbit anti-human VWF antibody (Dako). Following washing, HRP substrate 3,3′,5,5′-tetramethylbenzidine (Substrate Reagent Pack, R&D Systems, Abingdon, United Kingdom) was added, and the reaction subsequently stopped with 50 μL 1 M H2SO4. Absorbance was read at 450 nM using a VERSAmax microplate reader (Molecular Devices, Winnersh, United Kingdom). Normal plasma pooled from 20 WT C57BL/6J mice was used throughout as normal reference plasma. Plasma VWF:Ag levels in infected mice were expressed as a percentage of normal baseline murine VWF:Ag levels.
Similarly, VWF collagen-binding activity (VWF:CB) was also determined using a previously described ELISA.17,36 Briefly, 96-well microtiter plates (Thermo Scientific) were coated with type III collagen derived from human placenta (Sigma-Aldrich) at a final concentration of 40 µg/mL in sodium carbonate/bicarbonate buffer (0.035 M NaHCO3, 0.015 M Na2CO3; pH 9.6). After washing with imidazole buffer (0.12 M NaCl, 0.02 M imidazole, 0.005 M citric acid; pH 7.3), the plates were blocked using 3% BSA in imidazole for 1 hour at room temperature. Following further washing with imidazole buffer, test samples were added and incubated for 2 hours at 37°C. After a final washing, bound VWF was detected using HRP-conjugated rabbit anti-human VWF antibody (Dako) as described previously. Plasma VWF:CB was expressed as a percentage of uninfected controls.
Plasma VWF multimer analysis
The multimeric structure of plasma VWF was analyzed by electrophoresis using 1.8% agarose gels prepared from SeaKem HGT(P) Agarose (Lonza, Rockland, ME) in separating buffer (200 mM tris(hydroxymethyl)aminomethane [Tris], 100 mM glycine, and 0.1% sodium dodecyl sulfate [SDS]; pH 9.0). Stacking gels (0.75% agarose) were prepared from the same agarose and stacking buffer (70 mM Tris, 5 mM EDTA, 0.1% SDS pH 6.7). All gels were run in a Bio-Rad mini-gel electrophoresis system, with an outer buffer of 50 mM Tris, 75 mM glycine, and an inner buffer of 100 mM Tris, 150 mM glycine 0.1% SDS for 150 minutes at 50 V. After electrophoresis, protein was transferred to polyvinylidene difluoride membranes (Immobilon-FL, Millipore, Billerica, MA), blocked with 5% BSA, and incubated with rabbit anti-human VWF antibody (Dako), as previously described.37 After thorough washing, membranes were finally incubated with goat anti-rabbit-HRP (Santa Cruz Biotechnology, Santa Cruz, CA). Bound antibody was detected using the SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Scientific, Dublin, Ireland). The membrane was then exposed to autoradiography (x-ray) film (Fujifilm; Fisher Scientific, Ireland) and the films were developed using the AGFA CP1000 automatic film-developing system (AGFA, Bonn, Germany). To objectively quantify differences in VWF multimer composition, densitometry of typical individual lanes was performed using ImageJ software (Image Processing and Analysis in Java; National Institutes of Health, Bethesda, MD), as previously described.17
Plasma VWF propeptide, angiopoietin 2, and ADAMTS13 activity levels
Murine plasma VWF:pp concentration was determined by ELISA assay using a combination of a mouse-specific 349.3 capture antibody, and HRP-linked 349.2 antibody.37 Both antibodies were kind gifts from Dr Bob Montgomery (Blood Research Institute, BloodCenter of Wisconsin, Milwaukee, WI). Mouse angiopoietin-2 (Ang-2) plasma levels were measured by commercial ELISA according to manufacturer’s instructions (R&D Systems Quantikine ELISA immunoassay; R&D Systems). Finally, plasma ADAMTS13 activity was also assessed using a commercial FRETS-VWF73 assay (Peptides International Inc, Louisville, KY), as previously described.17
Blood–brain barrier permeability
Blood–brain barrier (BBB) permeability in WT and VWF−/− mice was investigated at baseline before inoculation with P berghei using Evans blue dye. In addition, BBB permeability was repeated at day +5 following P berghei infection. Briefly, in keeping with previous studies,38,39 2% Evans blue dye (Sigma-Aldrich) in phosphate-buffered saline was infused via lateral tail vein injection and then allowed to circulate for 1 hour. Mice were anesthetized with an intraperitoneal injection of 2.5% tribromethanol, and 40mL of normal saline was cardiacally perfused. To quantify extravasation of Evans blue across the BBB, murine brains were removed and weighed. Cerebral tissues were then homogenized and incubated in 50% trichloroacetic acid. Following centrifugation (14 000 rpm for 30 minutes at 4°C), supernatants were plated on a 96-well plate and absorbance was measured at 620 nm. Results were quantified according to a standard curve and expressed as nanogram Evans blue/nanogram brain.
Data presentation and statistical analysis
All experimental data and statistical analysis were performed using the GraphPad Prism program (GraphPad Prism, version 5.0 for Windows; GraphPad Software, Inc; San Diego, CA). Data were expressed as mean values ± standard error of the mean (SEM). To assess statistical differences, data were analyzed using Student unpaired 2-tailed Student t test. ECM clinical scoring data were assessed by 2-way analysis of variance. Finally, mouse survival data were compared using a log-rank (Mantel-Cox) test. For all statistical tests, P values <.05 were considered significant.
Results
Increased plasma VWF:Ag and VWF:CB levels in ECM
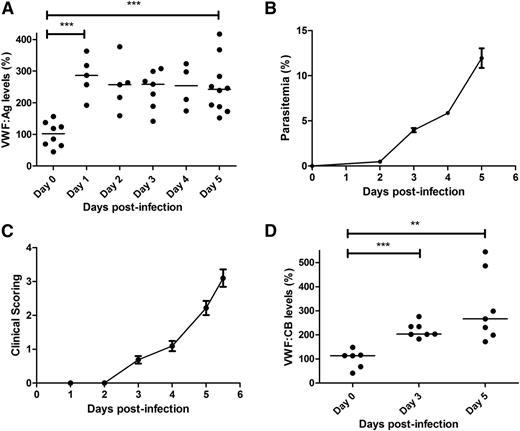
To further investigate the role of VWF in malaria pathogenesis, we used a previously described mouse model of ECM, in which C57BL/6J mice are infected with P berghei ANKA.40,41 In keeping with previous findings in children with severe P falciparum malaria,16,17 we observed a significant increase in plasma VWF:Ag levels in WT C57BL/6J mice following P berghei infection (Figure 1A). By day +5 after inoculation, median plasma VWF:Ag levels were increased approximately 2.4-fold (101.9% at day 0 vs 242.3% at day +5; P < .001), which was similar in magnitude to the increase in plasma VWF:Ag levels observed in children with CM.16 Interestingly, a significant increase in plasma VWF:Ag levels was already evident within 24 hours following P berghei inoculation. This early increase in plasma VWF levels occurred before the appearance of significant blood parasitemia levels (Figure 1B) and before the onset of clinical signs (Figure 1C). Again in keeping with our previous findings in children infected with P falciparum,17 plasma VWF:CB levels were also significantly elevated in C57BL/6J mice following P berghei infection (113.7% at day 0 vs 266.6% at day +5; P < .01) (Figure 1D). Furthermore, the magnitude of the increase in VWF:CB observed in the murine ECM model was again similar to that previously observed in patients with severe malaria. In contrast to the significant increases in plasma VWF:Ag and VWF:CB levels, we observed no significant increase in plasma VWF:pp levels in C57BL/6J mice following P berghei infection (supplemental Figure 1A, available on the Blood Web site). The molecular mechanism(s) underlying the observation that the plasma VWFpp:VWFAg ratio is not increased in the murine model of ECM remains unclear, but likely relates at least in part to the fact that the plasma half-life of VWF:pp is reduced in C57BL/6J mice (supplemental Figure 1B).
Plasma VWF:Ag and VWF:CB levels are increased in ECM. Following intraperitoneal inoculation with 2 × 106P berghei ANKA parasites, whole blood samples were collected from WT C57BL/6J mice by cardiac puncture. (A) Plasma VWF:Ag levels were then measured at each time point by ELISA. All ELISAs were performed in triplicate, and results presented represent the mean values ± SEM unless otherwise stated (*P < .05, **P < .01, ***P < .0001, respectively). (B) Peripheral blood P berghei parasitemia levels were determined from Giemsa-stained smears (n = 16; mean values shown). (C) ECM progression was monitored using a previously validated clinical scoring algorithm (n = 16; mean values shown). (D) Plasma VWF:CB activity levels were also determined by ELISA, as detailed in the “Materials and methods” section.
Plasma VWF:Ag and VWF:CB levels are increased in ECM. Following intraperitoneal inoculation with 2 × 106P berghei ANKA parasites, whole blood samples were collected from WT C57BL/6J mice by cardiac puncture. (A) Plasma VWF:Ag levels were then measured at each time point by ELISA. All ELISAs were performed in triplicate, and results presented represent the mean values ± SEM unless otherwise stated (*P < .05, **P < .01, ***P < .0001, respectively). (B) Peripheral blood P berghei parasitemia levels were determined from Giemsa-stained smears (n = 16; mean values shown). (C) ECM progression was monitored using a previously validated clinical scoring algorithm (n = 16; mean values shown). (D) Plasma VWF:CB activity levels were also determined by ELISA, as detailed in the “Materials and methods” section.
EC activation is a feature of ECM
Acute EC activation plays a critical role in the pathogenesis of P falciparum malaria.16,17,42,43 The marked increase in plasma VWF:Ag and VWF:CB observed following P berghei infection suggests that EC activation is also an early feature in mice before the development of CM. Ang-2 is another glycoprotein stored within WP bodies and secreted into plasma following EC activation.44 In addition, significantly elevated plasma Ang-2 levels have been reported in patients with severe P falciparum malaria, where Ang-2 levels were also shown to correlate with clinical outcome.18,19,45-47 In keeping with human data, a marked increase in murine plasma Ang-2 levels was observed in C57BL/6J mice following P berghei infection (Figure 2). For example, plasma Ang-2 levels by day +3 following P berghei infection were 56.8 ng/mL vs only 24.1 ng/mL at day 0 (P < .001). Collectively, these data confirm that marked EC activation and WP body exocytosis, which together represent early hallmarks of P falciparum malaria infection in human patients, also constitute prominent features in the murine model of ECM.
Early EC activation is a feature of ECM. Ang-2 is another protein stored within endothelial cell WP bodies. To further assess WP body exocytosis in WT C57BL/6J mice following P berghei infection, plasma Ang-2 levels were measured at specified time points using a commercial ELISA.
Early EC activation is a feature of ECM. Ang-2 is another protein stored within endothelial cell WP bodies. To further assess WP body exocytosis in WT C57BL/6J mice following P berghei infection, plasma Ang-2 levels were measured at specified time points using a commercial ELISA.
Severe P berghei malaria influences plasma VWF multimer composition
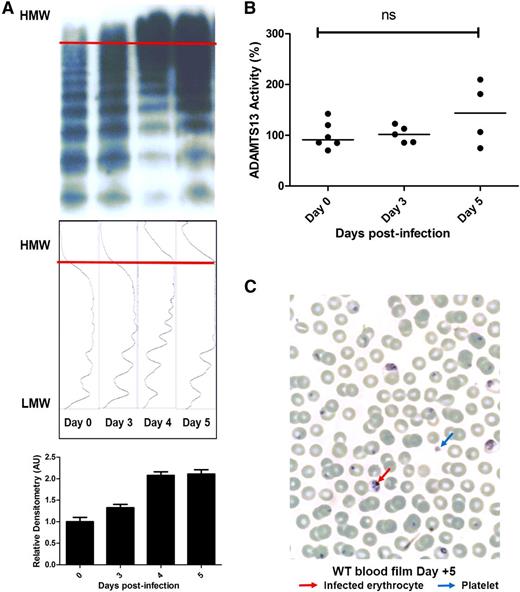
In addition to markedly elevated VWF:Ag and VWF:CB activity levels, pathological UL-VWF multimers have been reported in the plasma of children with CM.17 Consequently, VWF multimer analysis and densitometry were assessed in C57BL/6J mice following P berghei infection. In keeping with our previous findings in children, accumulation of abnormal UL-VWF multimers in the plasma was also a feature of murine ECM (Figure 3A; supplemental Figure 2). UL-VWF multimers were not present in the plasma of uninfected mice, but were observed from day +3 following P berghei infection. Normally, UL-VWF multimers secreted from WP bodies following EC activation are rapidly proteolyzed by the zinc metalloprotease ADAMTS13.48 Previous studies have reported modest reductions in plasma ADAMTS13 activity levels in children with P falciparum infection.17,21,22 In contrast, we observed no significant change in murine plasma ADAMTS13 activity following P berghei infection (Figure 3B). In addition, despite the presence of circulating UL-VWF, peripheral blood film examination demonstrated no significant features of microangiopathic hemolytic anemia (Figure 3C). In summary, these findings demonstrate that marked elevations of plasma VWF levels, coupled with the appearance of pathological UL-VWF multimers in the plasma, represent consistent features in human and experimental murine CM.
Severe P berghei malaria influences plasma VWF multimer composition. (A) Plasma VWF multimer distribution during the course of malaria infection was assessed by nonreducing agarose gel electrophoresis. Typical multimer gels from example mice are presented. From day +3 following P berghei infection, abnormal UL-VWF multimers were consistently observed in murine plasma. Densitometric scanning of multimer gels was also performed, with individual panels corresponding to each lane. The horizontal axis is optical density. Abnormal UL-VWF multimers are present from day +3 through day +5 (highlighted by bands beyond the arbitrary red line) that are not present before P berghei infection. (B) Plasma ADAMTS13 activity levels were measured by FRETS-VWF73 proteolysis assay. All assays were performed in triplicate, and results represent the mean values. NS, not significant. (C) Typical Giemsa-stained peripheral blood film from a WT C57BL/6J mouse at day +5 following P berghei infection. Infected erythrocytes and thrombocytopenia are evident, but there are no significant features of microangiopathic hemolytic anemia. AU, arbitrary unit; HMW, high molecular weight; LMW, low molecular weight.
Severe P berghei malaria influences plasma VWF multimer composition. (A) Plasma VWF multimer distribution during the course of malaria infection was assessed by nonreducing agarose gel electrophoresis. Typical multimer gels from example mice are presented. From day +3 following P berghei infection, abnormal UL-VWF multimers were consistently observed in murine plasma. Densitometric scanning of multimer gels was also performed, with individual panels corresponding to each lane. The horizontal axis is optical density. Abnormal UL-VWF multimers are present from day +3 through day +5 (highlighted by bands beyond the arbitrary red line) that are not present before P berghei infection. (B) Plasma ADAMTS13 activity levels were measured by FRETS-VWF73 proteolysis assay. All assays were performed in triplicate, and results represent the mean values. NS, not significant. (C) Typical Giemsa-stained peripheral blood film from a WT C57BL/6J mouse at day +5 following P berghei infection. Infected erythrocytes and thrombocytopenia are evident, but there are no significant features of microangiopathic hemolytic anemia. AU, arbitrary unit; HMW, high molecular weight; LMW, low molecular weight.
VWF-deficient mice are protected against ECM
To determine whether increased plasma VWF levels represent simply a biomarker of acute EC activation in ECM, or whether elevated plasma VWF and/or UL-VWF multimers are directly involved in mediating the pathogenesis of ECM, we studied P berghei infection in VWF−/− mice compared with WT. Using a previously validated clinical scoring algorithm to assess experimental malaria progression,31 we found that the clinical features of ECM progressed significantly more slowly in the VWF−/− mice compared with WT mice (P = .001) (Figure 4A). Importantly however, VWF−/− mice still developed typical neurological signs, and died of ECM rather than severe anemia. In addition, overall survival was significantly prolonged in VWF−/− mice compared with WT mice (P = .01) (Figure 4B). For example, whereas only approximately 30% of WT mice survived until day +6 following P berghei infection, more than 80% of the VWF−/− mice remained alive.
VWF-deficient mice are significantly protected against ECM. To investigate whether VWF may be directly involved in the pathogenesis of ECM, P berghei infection in VWF−/− mice was investigated. (A) Following inoculation with P berghei, clinical phenotype and progression in VWF−/− mice (n = 10) and WT C57BL/6J mice (n = 16) were compared using a validated clinical scoring algorithm. Results presented represent the mean values ± SEM (*P < .05, **P < .01, ***P < .0001, respectively). (B) In addition, overall survival in WT (n = 16) and VWF−/− (n = 10) mice infected with 2 × 106P berghei parasites was also determined and analyzed by log-rank (Mantel-Cox) test. (C) Because VWF plays a critical role in modulating platelet adhesion and aggregation, sequential platelet counts were performed in VWF−/− mice and WT C57BL/6J mice following P berghei infection (n = 4-5 mice per time point). (D) Peripheral blood P berghei parasitemia levels were determined following inoculation in both VWF−/− (n = 10) and WT C57BL/6J mice (n = 10) at specified time points from Giemsa-stained smears. Results presented represent the mean values ± SEM.
VWF-deficient mice are significantly protected against ECM. To investigate whether VWF may be directly involved in the pathogenesis of ECM, P berghei infection in VWF−/− mice was investigated. (A) Following inoculation with P berghei, clinical phenotype and progression in VWF−/− mice (n = 10) and WT C57BL/6J mice (n = 16) were compared using a validated clinical scoring algorithm. Results presented represent the mean values ± SEM (*P < .05, **P < .01, ***P < .0001, respectively). (B) In addition, overall survival in WT (n = 16) and VWF−/− (n = 10) mice infected with 2 × 106P berghei parasites was also determined and analyzed by log-rank (Mantel-Cox) test. (C) Because VWF plays a critical role in modulating platelet adhesion and aggregation, sequential platelet counts were performed in VWF−/− mice and WT C57BL/6J mice following P berghei infection (n = 4-5 mice per time point). (D) Peripheral blood P berghei parasitemia levels were determined following inoculation in both VWF−/− (n = 10) and WT C57BL/6J mice (n = 10) at specified time points from Giemsa-stained smears. Results presented represent the mean values ± SEM.
Thrombocytopenia in ECM is independent of plasma VWF
Significant thrombocytopenia is common feature in patients with P falciparum malaria.49,50 This thrombocytopenia is predominantly the result of increased platelet clearance.24,51 A putative role for UL-VWF in contributing to this enhanced platelet clearance has been proposed.23,24 Furthermore, recent studies have identified novel roles for platelets in killing intraerythrocytic malaria parasites52 and in modulating host immune responses during the early stages of P falciparum infection.53 Consequently, to investigate potential mechanisms through which VWF−/− mice were protected against ECM, daily platelet counts were performed in WT and VWF−/− mice following P berghei infection. In keeping with observations in human patients, significant thrombocytopenia was a consistent feature in this murine model (Figure 4C). At day +4 following inoculation, mean platelet count had fallen by approximately 70% in WT mice. Interestingly, significant thrombocytopenia was also observed in VWF−/− mice infected with P berghei (Figure 4C). Importantly, despite the differences in clinical malaria progression, no significant differences in platelet counts were observed between VWF−/− and WT mice. Finally, we observed no significant differences in blood P berghei parasitemia levels between VWF−/− mice and WT mice, respectively (Figure 4D).
ECM-induced BBB permeability is attenuated in VWF-deficient mice
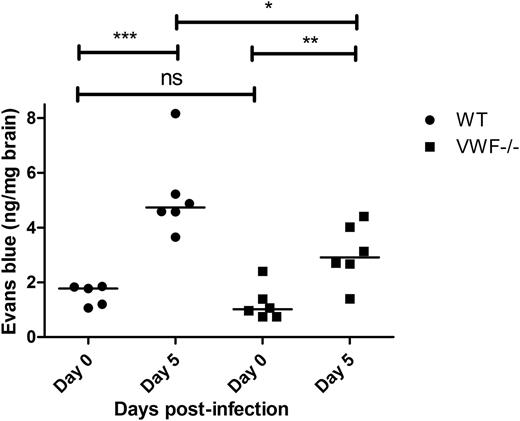
VWF is expressed abundantly by cerebral ECs.54 In addition, endothelial VWF has recently been shown to play an important role in modulating permeability of the BBB under different pathological conditions.38,39 Although the underlying biological mechanisms remain poorly understood, enhanced permeability of the BBB has also been implicated in the pathogenesis of CM.55-58 We therefore investigated BBB permeability in VWF−/− mice compared with WT mice following infection with P berghei. In keeping with previous studies, we observed no significant difference in BBB permeability to Evans blue dye between VWF−/− and WT mice before P berghei inoculation (Figure 5). By day +5 following infection, BBB permeability was markedly elevated in WT (1.8 ng/mg at day 0 vs 4.7 ng/mg at day +5; P < .001). Interestingly, although BBB was also affected in VWF−/− mice at day +5, the increase in permeability was significantly lower than that observed in WT mice (P < .05).
ECM-induced BBB permeability is attenuated in VWF-deficient mice. BBB permeability was assessed following a lateral tail vein infusion of Evans blue dye. Because Evan’s blue binds to murine albumin, it does not normally cross an intact BBB. Before P berghei inoculation (day 0), baseline BBB permeability was determined in WT C57BL/6J mice (black circles; n = 5) and compared with that of VWF−/− mice (blue squares; n = 6). Subsequently, BBB permeability was then reassessed in WT C57BL/6J mice (black circles; n = 6) and VWF−/− mice (blue squares; n = 6) at day +5 following P berghei infection. NS, not significant.
ECM-induced BBB permeability is attenuated in VWF-deficient mice. BBB permeability was assessed following a lateral tail vein infusion of Evans blue dye. Because Evan’s blue binds to murine albumin, it does not normally cross an intact BBB. Before P berghei inoculation (day 0), baseline BBB permeability was determined in WT C57BL/6J mice (black circles; n = 5) and compared with that of VWF−/− mice (blue squares; n = 6). Subsequently, BBB permeability was then reassessed in WT C57BL/6J mice (black circles; n = 6) and VWF−/− mice (blue squares; n = 6) at day +5 following P berghei infection. NS, not significant.
Discussion
C57BL/6 mice infected with P berghei ANKA typically develop a complex neurological syndrome involving clinical features similar to those observed in human CM.40,41,59 These include ataxia, paralysis, seizures, and coma. Depending upon the dose of P berghei, infected mice typically die within 6 to 10 days. Although there are important differences between human CM and the ECM model,60,61 both P falciparum and P berghei infections are associated with pronounced effects on EC biology.40,41,62,63 In this study, we demonstrate that P berghei infection mirrors the activated EC phenotype observed in African children with severe P falciparum malaria, with significant increases in plasma VWF:Ag and VWF:CB levels.16-20 In addition, and in keeping with previous data from human volunteers infected with P falciparum,23 these increased plasma VWF levels were observed from a very early stage following P berghei infection. Thus, the early acute EC activation and WP body secretion that constitute hallmarks of P falciparum infection in humans are replicated in this ECM model. Interestingly, P falciparum–infected humans and P berghei–infected mice both exhibit increased plasma VWF levels before the number of IEs had reached 0.1%. The biological mechanism underlying this very early EC activation remains unknown64 ; however, further studies using this murine model may enable definition of the biological mechanisms through which early EC activation in response to malaria infection is modulated.
Previous studies have demonstrated that severe P falciparum malaria is associated with accumulation of abnormal UL-VWF multimers in the plasma.17,21 In this study, we further demonstrate that plasma UL-VWF multimers also constitute a feature of the murine model of ECM. The molecular mechanisms responsible for this UL-VWF accumulation have not been defined. However, marked exocytosis of UL-VWF multimers from WP bodies following acute EC activation is likely to represent an important contributing factor. Nevertheless, UL-VWF multimers secreted from WP bodies typically undergo rapid proteolysis by ADAMTS13 on EC surfaces. Following P berghei infection, we observed no significant reduction in murine ADAMTS13 activity as assessed by FRETS-VWF75 assay. In contrast, previous studies did report a mild reduction in plasma ADAMTS13 activity (using both FRETS-VWF75 and full-length VWF cleavage assays) in children and adult patients with severe malaria compared with healthy controls.17,21,22 Importantly, however, residual plasma ADAMTS13 levels in African children with CM still remained higher than 60%, which should be sufficient to prevent the accumulation of hyperreactive UL-VWF multimers.17 In summary, therefore, it seems likely that the ability of ADAMTS13 to cleave VWF in vivo is reduced in both humans and mice with CM. Although several putative inhibitors of ADAMTS13 have been identified,65-69 we previously showed that those described to date could not explain the accumulation of UL-VWF multimers observed in children with CM.17 Furthermore, this combination of UL-VWF multimers with normal plasma ADAMTS13 levels has also recently been reported in other conditions, including patients with sickle cell disease.70 Further studies to investigate how UL-VWF multimers accumulate in the murine model of ECM despite the presence of ADAMTS13 may therefore be useful in elucidating novel molecular mechanisms involved in both the physiological and pathological regulation of ADAMTS13 activity in vivo. Interestingly, different murine strains demonstrate significant variability in susceptibility to malaria infection.41,71 The genetic modifiers responsible for this interstrain variation remain unclear, but plasma VWF:Ag levels vary markedly between different in-bred mouse strains.72 In addition, plasma ADAMTS13 activity levels are reduced in some strains (including C57BL/6) because the insertion of a retrotransposon element into the ADAMTS13 gene.73,74
Given the key role played by VWF in modulating platelet adhesion and aggregation, together with the accumulating data demonstrating that platelet-EC interactions play a critical role in malaria biology,25-27 we hypothesized that VWF may play a novel role in modulating the pathophysiology of malaria. This hypothesis was supported by previous in vitro experiments in which we showed that platelet-decorated UL-VWF strings could recruit P falciparum IE to EC surfaces under shear stress conditions.28 In this study, we demonstrate that VWF−/− mice are significantly protected against P berghei infection compared with WT controls. This survival difference was not explained by a difference in P berghei parasitemia levels, which were similar in both VWF−/− and WT mice. Significant thrombocytopenia is a common feature of both human and murine malaria and has been attributed to a number of mechanisms, including sequestration and enhanced clearance by the spleen.49-51 Moreover, recent studies have described a role for platelets in modulating the innate host defense during the early stages of malaria infection.52,53 Interestingly, although previous studies suggested that the UL-VWF might be important in mediating this malaria-associated thrombocytopenia,23,24 we observed no significant difference in P berghei–induced thrombocytopenia in VWF−/− compared with WT mice. Collectively, these data suggest that enhanced platelet clearance in ECM is not influenced by the elevated plasma VWF:Ag levels, nor by circulating UL-VWF multimers. In addition, VWF−/− mice are clearly protected against ECM through a platelet-independent mechanism.
Severe malaria infection has been associated with enhanced permeability of the BBB.55-57 In addition, recent studies have demonstrated that VWF plays an important role in regulating the BBB.38,39 For example, significantly attenuated BBB permeability was observed in VWF−/− mice compared with WT controls in a hypoxia/reoxygenation model, and also in an experimental model of status epilepticus.38 Although the molecular mechanisms through which VWF influences BBB integrity remain poorly understood, a significant increase in expression of the endothelial tight junction protein claudin-5 was demonstrated in the cerebral microvascular EC of VWF−/− mice compared with WT controls.38 In this study, we found that P berghei infection was associated with a significant increase in BBB permeability in both WT and VWF−/− mice. Interestingly, however, the degree of ECM-associated enhanced BBB permeability was significantly attenuated in the VWF−/− mice compared with WT controls. Further studies will be required to fully elucidate the mechanisms through which VWF protects BBB permeability and to determine the importance of this BBB effect in modulating the improved ECM survival observed in VWF−/− mice.
VWF synthesis is essential for the normal formation of WP bodies within EC.75 Consequently, VWF−/− mice are not only deficient in plasma and platelet VWF, but also do not possess WP bodies.29 Importantly, in addition to VWF and VWF:pp, WP bodies also normally store other proteins including P-selectin, Ang-2, and osteoprotegerin. Whether the absence of WP bodies may be important in attenuating ECM in VWF−/− mice remains unclear. However, previous studies with an inhibitory P-selectin aptamer suggested that P-selectin does not play a major role in modulating the enhanced BBB protection observed in VWF−/− mice.38 In this study we have shown that plasma Ang-2 levels are also significantly increased in murine ECM. This observation is consistent with previous data from children with severe P falciparum malaria.18,19,45-47 Interestingly, Ang-2 has also been shown to function as an autocrine regulator by sensitizing EC to activation by tumor necrosis factor,76 and thus may play a role in modulating the early acute EC activation associated with both P falciparum and P berghei infections. In addition, Ang-2 has also been reported to influence BBB permeability.44,76 Despite the absence of WP bodies, baseline plasma Ang-2 levels in the VWF−/− mice were not significantly different compared with those in WT C57BL/6 controls (mean, 23.1 ± 5.4 vs 22.8 ± 3.2 ng/mL, respectively).
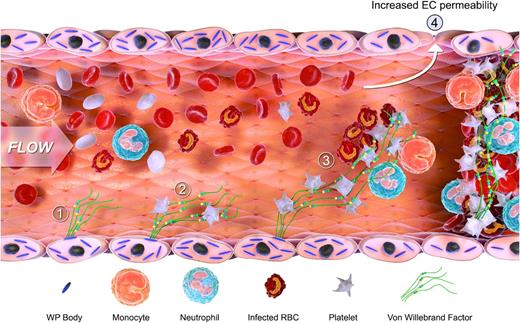
In conclusion, we demonstrate that early significant EC activation represents a consistent feature of the murine ECM model. In keeping with our previous observations in children with severe P falciparum malaria, this EC activation results in a marked increase in plasma VWF levels, together with a pathological accumulation of hyperreactive UL-VWF multimers in plasma. Although the pathobiology underlying CM remains poorly understood, it is complex and involves multiple different mechanisms (including EC activation, IE sequestration; platelet and leukocyte recruitment; cytokine secretion; innate and adaptive immune responses; alteration in EC and BBB permeability). Given this complexity, we postulate that VWF may influence malaria pathogenesis through several different mechanisms (Figure 6). For example, during the early stages following malaria infection, formation of UL-VWF strings on activated EC surfaces may be important in recruiting platelets. Subsequently, platelet-bound VWF may play a role in modulating microvasculature sequestration by recruiting malaria-IEs, and also by binding to granulocytes and activated monocytes, respectively.77,78 In addition, VWF may also be involved in the later stages of CM pathogenesis. For example, VWF plays an important role in regulating leukocyte extravasation and, as we have shown, also influences BBB permeability in CM. Defining the roles of VWF and/or UL-VWF multimers in this setting may offer novel therapeutic opportunities.
Schematic diagram illustrating the proposed mechanisms through which VWF is involved in malaria pathogenesis. EC activation and release of WP body contents constitute common early features in both human and murine malaria. This results in the secretion in the release of UL-VWF multimers into the plasma (1) and a marked increase in plasma VWF levels. VWF may influence malaria pathogenesis through several different mechanisms. First, UL-VWF strings on the surface of activated EC recruit and sequester platelets within the microvasculature (2). These tethered platelets cause further EC activation, and thus more WP body secretion. In addition, the platelet-decorated VWF may be important in modulating further sequestration by recruiting both malaria-infected erythrocytes and by binding to granulocytes and activated monocytes respectively (3). The VWF-mediated sequestration of platelets, infected erythrocytes, and leukocytes leads to further enhanced EC activation. During the later stages of CM pathogenesis, VWF may be important in regulating EC permeability, BBB permeability, and leukocyte extravasation (4). Finally, given that platelets play a critical role in the development of microvasculature occlusion, we postulate that VWF may also be important in this context (5).
Schematic diagram illustrating the proposed mechanisms through which VWF is involved in malaria pathogenesis. EC activation and release of WP body contents constitute common early features in both human and murine malaria. This results in the secretion in the release of UL-VWF multimers into the plasma (1) and a marked increase in plasma VWF levels. VWF may influence malaria pathogenesis through several different mechanisms. First, UL-VWF strings on the surface of activated EC recruit and sequester platelets within the microvasculature (2). These tethered platelets cause further EC activation, and thus more WP body secretion. In addition, the platelet-decorated VWF may be important in modulating further sequestration by recruiting both malaria-infected erythrocytes and by binding to granulocytes and activated monocytes respectively (3). The VWF-mediated sequestration of platelets, infected erythrocytes, and leukocytes leads to further enhanced EC activation. During the later stages of CM pathogenesis, VWF may be important in regulating EC permeability, BBB permeability, and leukocyte extravasation (4). Finally, given that platelets play a critical role in the development of microvasculature occlusion, we postulate that VWF may also be important in this context (5).
Presented in abstract form as an oral presentation at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, December 2014.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Children’s Medical and Research Foundation, Our Lady’s Children’s Hospital, Crumlin (O.P.S., A.C., and J.S.O.), and through a Science Foundation Ireland Principal Investigator Award (11/PI/1066, J.S.O.)
Authorship
Contribution: N.O., K.G., J.M.O., N.D., T.M.B., U.B., G.E.G., S.M., and A.C. performed experiments; N.O., K.G., J.M.O., T.M.B., P.G.F., U.B., G.E.G., R.J.S.P., O.P.S., A.G.C., and J.S.O. designed the research and analyzed the data. All authors were involved in writing and reviewing the paper.
Conflict-of-interest disclosure: J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, and Octapharma; on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, and Pfizer; and has also received research grant funding awards from Baxter, Bayer, Pfizer, and Novo Nordisk. The remaining authors declare no competing financial interests.
Correspondence: James O’Donnell, Haemostasis Research Group, Institute of Molecular Medicine, Trinity College Dublin, Ireland; e-mail: jodonne@tcd.ie.
References
Author notes
N.O. and K.G. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal