Key Points
Murine neutrophils can be stimulated by LPS to express de novo ICAM-1 in vitro and in murine models of endotoxemia in vivo.
Neutrophil ICAM-1 expression correlated with enhanced phagocytosis and ROS generation, and ICAM-1 deficiency caused defective phagocytosis.
Abstract
Intracellular adhesion molecule-1 (ICAM-1) is a transmembrane glycoprotein expressed on the cell surface of numerous cell types such as endothelial and epithelial cells, vascular smooth muscle cells, and certain leukocyte subsets. With respect to the latter, ICAM-1 has been detected on neutrophils in several clinical and experimental settings, but little is known about the regulation of expression or function of neutrophil ICAM-1. In this study, we report on the de novo induction of ICAM-1 on the cell surface of murine neutrophils by lipopolysaccharide (LPS), tumor necrosis factor, and zymosan particles in vitro. The induction of neutrophil ICAM-1 was associated with enhanced phagocytosis of zymosan particles and reactive oxygen species (ROS) generation. Conversely, neutrophils from ICAM-1–deficient mice were defective in these effector functions. Mechanistically, ICAM-1–mediated intracellular signaling appeared to support neutrophil ROS generation and phagocytosis. In vivo, LPS-induced inflammation in the mouse cremaster muscle and peritoneal cavity led to ICAM-1 expression on intravascular and locally transmigrated neutrophils. The use of chimeric mice deficient in ICAM-1 on myeloid cells demonstrated that neutrophil ICAM-1 was not required for local neutrophil transmigration, but supported optimal intravascular and extravascular phagocytosis of zymosan particles. Collectively, the present results shed light on regulation of expression and function of ICAM-1 on neutrophils and identify it as an additional regulator of neutrophil effector responses in host defense.
Introduction
Intracellular adhesion molecule-1 (ICAM-1; CD54) is a member of the immunoglobulin (Ig)-like gene superfamily composed of an extracellular domain containing 5 Ig-like structures, a transmembrane domain, and a short cytoplasmic tail of 28 amino acids.1-3 It is a key adhesion molecule with significant signaling properties that has been associated with numerous cellular responses such as cell adhesion, migration, and aggregation.4-6 ICAM-1 is constitutively expressed on the surface of a wide range of cells, including endothelial and epithelial cells, smooth muscle cells, pericytes, fibroblasts, and keratinocytes.4,5,7-9 The expression of ICAM-1 is primarily regulated in a transcriptional manner, notably by inflammatory stimuli such as the cytokines interleukin-1β (IL-1β), tumor necrosis factor (TNF), and lipopolysaccharide (LPS).4,9,10 For example, these stimuli can enhance the constitutive expression of endothelial cell ICAM-1 and promote leukocyte-endothelial cell adhesion and trafficking via interactions of ICAM-1 with its key leukocyte ligands, the integrins LFA-1 and Mac-1.8,11,12 Ligation of endothelial cell ICAM-1 can trigger elevations in cytoplasmic Ca2+, activation of myosin contractility, protein kinase C, and of the small guanosine triphosphatases (eg, Rho, Rac, Rap1).6,10 Several cytosolic and adaptor proteins, such as α-actinins, ezrin, cortactin, and filamin B, also interact with the C-terminal domain of endothelial cell ICAM-1 and contribute to localized cytoskeletal rearrangements and leukocyte-endothelial interactions.13 How such events are linked in endothelial cells, and details of ICAM-1 signaling in different cell types are not fully understood, but it is generally accepted that the cytoplasmic tail of the molecule plays a key role in supporting ICAM-1–mediated responses.
In addition to being expressed on tissue-resident cells, ICAM-1 is expressed on most leukocyte subsets, such as activated lymphocytes and monocytes. The best documented role of leukocyte ICAM-1 is in the formation of immune synapses between T cells and antigen-presenting or natural killer cells and their targets.14 These responses are primarily mediated by ICAM-1–LFA-1 interactions.14 With respect to neutrophils, ICAM-1 is generally absent or expressed at very low levels on circulating blood cells in humans and mice,15-18 although elevated levels have been reported in a limited number of clinical and experimental scenarios. For example, ICAM-1 has been detected on blood and peritoneal neutrophils in patients with bacterial peritonitis,19 on blood and nasopharyngeal aspirated neutrophils in infants with respiratory syncytial virus,20 on blood neutrophils after low-dose intravenous endotoxin,21 and on blood and bronchoalveolar lavage neutrophils from septic and sarcoidosis patients.22 In animal models, immunization of mice with Pseudomonas aeruginosa can also upregulate neutrophil ICAM-1 in vivo,18 and neutrophil reverse transendothelial cell migration has been associated with increased neutrophil ICAM-1 expression in vitro and in vivo with respect to human and mouse neutrophils.17,23 Furthermore, there are indications that granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor, LPS, TNF, bacterial lipoprotein, and Staphylococcus aureus can induce expression of ICAM-1 on human neutrophils in vitro.16,24-27 In terms of its physiological role, neutrophil ICAM-1 has been linked to cellular aggregation, increased generation of reactive oxygen species (ROS)26,28 and in supporting interactions with other components of the immune system.21 Despite these scant reports, little is known about the mechanisms through which ICAM-1 is expressed on neutrophils and its potential pathophysiological functions. In the present study, we demonstrate the ability of murine neutrophils to upregulate ICAM-1 in a stimulus-specific manner both in vitro and in vivo, with LPS being identified as an effective inducer of neutrophil ICAM-1 messenger RNA (mRNA). Functionally, neutrophil ICAM-1 was found to be important for enhanced neutrophil effector functions, with ICAM-1–deficient neutrophils exhibiting significantly reduced levels of phagocytosis in vitro and in murine models of endotoxemia. The mechanism through which neutrophil ICAM-1supported phagocytosis appeared to be linked to ICAM-1–fibrinogen interactions and ICAM-1–mediated intracellular signaling involving the tyrosine kinase Syk. Collectively, the present findings shed significant light on the regulation and function of neutrophil ICAM-1 and suggest that in addition to its expression on endothelial cells and lymphocytes, neutrophil ICAM-1 contributes to the host’s defense mechanism against pathogens.
Materials and methods
For detailed methodology, see the supplemental Materials on the Blood Web site.
Animals
Male wild-type (WT), ICAM-1–deficient (KO)29 or heterozygous mice with green fluorescent protein expressed under the lysozyme-M promoter (LysM-eGFP)30 mice on a C57BL/6 background were used. LysM-eGFP mice are referred to as WT-LysM-eGFP, to distinguish them from WT mice. All experiments were performed under the UK Home Office legislation for the protection of animals.
Generation of chimeric mice
A mixture of WT-LysM-eGFP and ICAM-1 KO bone marrow was injected IV to irradiated WT mice to create chimeric mice that expressed ICAM-1 on the vasculature with ICAM-1 KO or WT-LysM-GFP leukocytes.31
In vivo inflammatory models
Animals were injected intrascrotally (IS) or intraperitoneally (IP) with LPS or IL-1β and, after 4 hours, blood, peritoneal lavage, and tissue samples were collected. In some cases, fluorescent zymosan-Texas-Red (ZymTR) was injected (IP or IV) 15 minutes before tissue collection to quantify in vivo phagocytosis. Cremaster muscles and lungs were labeled with fluorescent monoclonal antibodies (mAbs) against PECAM-1 (endothelial cell junctions), S100a9 (neutrophils), lectin (vasculature), and ICAM-1, and analyzed by confocal microscopy. Leukocytes from blood, enzymatically digested lung tissue, or peritoneal lavage were analyzed by flow cytometry (see the following section).
Quantification of ROS and phagocytosis
Whole blood from WT or ICAM-1 KO mice was stimulated with a panel of inflammatory mediators for 4 hours. The ROS probe dihydrorhodamine-123 (DHR), ZymTR, and all pharmacological interventions were added to blood samples as detailed.
Flow cytometry
Blood, peritoneal lavage, or lung tissue digest leukocytes were labeled with the fluorescent dead cell nuclear marker 4,6 diamidino-2-phenylindole (DAPI) and fluorescent mAbs against CD45, Ly6G, ICAM-1, or isotype controls, or the ROS probe DHR or ZymTR before analysis by flow cytometry.
Confocal microscopy
Fluorescently labeled cremaster muscles, lung tissues, or isolated leukocytes were viewed using a Leica-SP5 confocal microscope incorporating a 20× water-dipping objective (NA 1.0). Quantifications were carried out using Imaris (Bitplane) or Leica LASF-Lite software.
Quantitative reverse transcription-polymerase chain reaction
Neutrophils were purified by fluorescence-activated cell sorter. The fold change in ICAM-1 mRNA was quantified using glyceraldehyde-3-phosphate dehydrogenase as a housekeeping gene.
Western blot
Naive or LPS-stimulated neutrophils were purified and treated with protease inhibitor before lysis. ICAM-1 and β-actin expression was detected by western blot.
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). Statistical significance was assessed by the Student t test or by 1-way analysis of variance (ANOVA) with Newman-Keuls multiple comparison test. P values below .05 were considered significant.
Results
ICAM-1 is upregulated on mouse neutrophils in a stimulus-specific manner in vitro
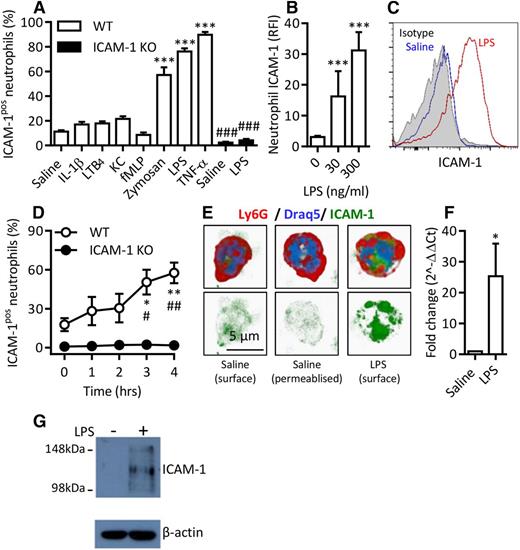
Although several studies have reported on the presence of ICAM-1 on neutrophils,19,20,22 little is known about the regulation of this expression. Analysis of mouse blood leukocytes by flow cytometry indicated a low percentage of ICAM-1–positive neutrophils. This was not changed following in vitro stimulation of whole blood with a number of neutrophil chemotactic stimuli (leukotriene B4 [LTB4], keratinocyte-derived chemokine [KC], and N-formyl-methionine-leucine-phenylalanine [fMLP]) or with the cytokine IL-1β (4 hours’ stimulation at 37°C for all mediators; Figure 1A). In contrast, LPS, TNF, and zymosan particles significantly increased the percentage of ICAM-1–expressing neutrophils (Figure 1A). The lack of ICAM-1 staining in samples from ICAM-1 KO mice confirmed the labeling specificity. Of note, WT and ICAM-1 KO neutrophils showed similar levels of enhanced CD18 expression in response to LPS (data not shown).
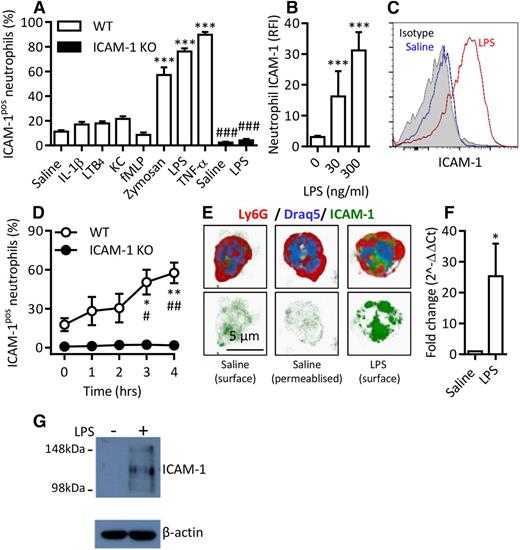
Murine neutrophils exhibit enhanced ICAM-1 expression in a stimulus-specific manner in vitro. Whole blood from WT or ICAM-1 KO mice was incubated (4 hours at 37°C) with IL-1β (50 ng/mL), LTB4 (30 nM), KC (30 nM), fMLP (1 µM), Zymosan (10 µg/mL), LPS (300 ng/mL), or TNF (100 ng/mL). After the red blood cell (RBC) lysis step, leukocytes were labeled with DAPI and fluorescent antibodies against CD45, Ly6G, and ICAM-1. Samples were analyzed by flow cytometry. (A) Percentage of ICAM-1–positive (ICAM-1pos) neutrophils in WT and ICAM-1 KO samples. (B) RFI compared with isotype control of unstimulated and LPS-stimulated WT neutrophils. (C) Representative histogram of ICAM-1 expression on saline or LPS-stimulated WT neutrophils compared with binding of an isotype control. (D) Time course of cell-surface ICAM-1 expression. (E) Naive or LPS-stimulated WT neutrophils labeled with fluorescent antibodies against Ly6G and ICAM-1 and the nuclear marker Draq5. Some samples were permeabilized before ICAM-1 labeling to visualize intracellular stores in addition to surface expression. Cells were imaged by confocal microscopy and analyzed using Imaris imaging software. (F) Quantitative polymerase chain reaction analysis of ICAM-1 mRNA in saline or LPS-stimulated neutrophils. Unstimulated expression levels were normalized to 1; LPS-stimulated data are shown as fold change compared with unstimulated samples. (G) Western blot of ICAM-1 and β-actin loading control in naive or LPS-stimulated purified neutrophils treated with protease inhibitor at the time of collection. Data are expressed as mean ± SEM of n = 3-27 animals/group. Statistically significant (t test) differences between treatment groups: *P < .05 and ***P < .001; differences between WT and ICAM-1 KO: #P < .05, ##P < .01, and ###P < .001.
Murine neutrophils exhibit enhanced ICAM-1 expression in a stimulus-specific manner in vitro. Whole blood from WT or ICAM-1 KO mice was incubated (4 hours at 37°C) with IL-1β (50 ng/mL), LTB4 (30 nM), KC (30 nM), fMLP (1 µM), Zymosan (10 µg/mL), LPS (300 ng/mL), or TNF (100 ng/mL). After the red blood cell (RBC) lysis step, leukocytes were labeled with DAPI and fluorescent antibodies against CD45, Ly6G, and ICAM-1. Samples were analyzed by flow cytometry. (A) Percentage of ICAM-1–positive (ICAM-1pos) neutrophils in WT and ICAM-1 KO samples. (B) RFI compared with isotype control of unstimulated and LPS-stimulated WT neutrophils. (C) Representative histogram of ICAM-1 expression on saline or LPS-stimulated WT neutrophils compared with binding of an isotype control. (D) Time course of cell-surface ICAM-1 expression. (E) Naive or LPS-stimulated WT neutrophils labeled with fluorescent antibodies against Ly6G and ICAM-1 and the nuclear marker Draq5. Some samples were permeabilized before ICAM-1 labeling to visualize intracellular stores in addition to surface expression. Cells were imaged by confocal microscopy and analyzed using Imaris imaging software. (F) Quantitative polymerase chain reaction analysis of ICAM-1 mRNA in saline or LPS-stimulated neutrophils. Unstimulated expression levels were normalized to 1; LPS-stimulated data are shown as fold change compared with unstimulated samples. (G) Western blot of ICAM-1 and β-actin loading control in naive or LPS-stimulated purified neutrophils treated with protease inhibitor at the time of collection. Data are expressed as mean ± SEM of n = 3-27 animals/group. Statistically significant (t test) differences between treatment groups: *P < .05 and ***P < .001; differences between WT and ICAM-1 KO: #P < .05, ##P < .01, and ###P < .001.
The effect of LPS was investigated further and shown to induce ICAM-1 on neutrophils in terms of relative fluorescence intensity (RFI) and also in a dose-dependent manner (Figure 1B-C). LPS-induced expression of ICAM-1 on neutrophils was time-dependent, and a significant effect was noted at 3 hours poststimulation (Figure 1D). The observed slow expression suggested that this ICAM-1 was not derived from preformed intracellular stores and indeed no evidence for intracellular expression of ICAM-1 in permeabilized unstimulated neutrophils was observed (Figure 1E). Quantitative reverse transcription-polymerase chain reaction analysis of unstimulated and LPS-stimulated purified neutrophils indicated low levels of ICAM-1 mRNA in unstimulated cells that was significantly elevated by LPS, providing evidence for de novo generation of ICAM-1 (Figure 1F). Western Blot analysis of unstimulated and LPS-stimulated neutrophils, treated with a protease inhibitor, confirmed that naive cells have low or absent levels of ICAM-1 (Figure 1G).
Collectively, these results demonstrate that murine neutrophils can be stimulated to express cell-surface ICAM-1 in a stimulus-specific manner via induction of ICAM-1 mRNA and de novo generation of ICAM-1 protein.
Neutrophil ICAM-1 expression facilitates enhanced neutrophil effector functions
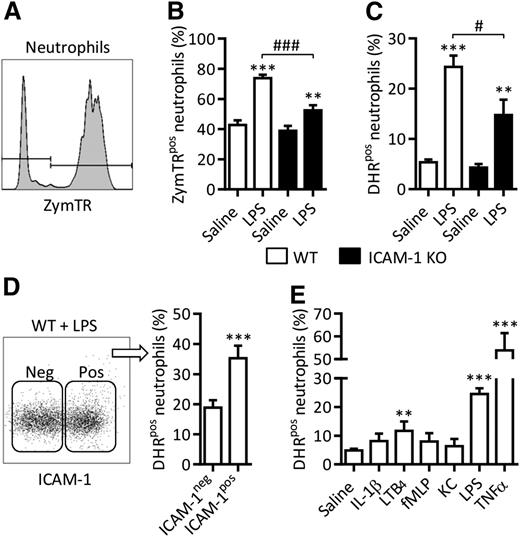
The potential functional implications of LPS-induced neutrophil ICAM-1 were next investigated. Because the ICAM-1 ligand Mac-1 is a key regulator of neutrophil phagocytosis, we hypothesized that neutrophil ICAM-1 might also facilitate this process. To address this possibility, neutrophil phagocytosis was quantified using ZymTR. LPS stimulation of WT blood samples significantly increased the percentage of ZymTR-positive neutrophils (ZymTRpos), as compared with saline-treated samples (Figure 2A-B). This response was significantly suppressed in samples from ICAM-1 KOs (Figure 2B). Use of an ImageStream platform, combining fluorescence microscopy with high-throughput flow cytometry, indicated that in both genotypes the majority of ZymTR-associated neutrophils had internalized the ZymTR particles within 15 minutes (ie, ∼97%), confirming that this assay quantifies phagocytosis of ZymTR, rather than surface decoration. Neutrophil ICAM-1 also appeared to support ROS generation. Specifically, LPS-stimulated WT samples showed a significantly higher DHR signal, as compared with saline-treated samples, and this was significantly reduced in samples from ICAM-1 KO mice (Figure 2C). Furthermore, ICAM-1–positive neutrophils exhibited a greater DHR signal than ICAM-1–negative cells (Figure 2D). Interestingly, across the panel of neutrophil stimuli used in Figure 1A, a correlation between ICAM-1 upregulation and ROS generation was noted with LPS and TNF inducing ICAM-1 expression and ROS generation, whereas IL-1β, fMLP and KC did not (Figure 2E). Collectively, these results demonstrate the involvement of neutrophil ICAM-1 in generation of intracellular ROS and report on a previously unknown function of neutrophil ICAM-1 as a facilitator of phagocytosis.
ICAM-1 expression facilitates enhanced neutrophil effector functions. Whole blood from WT or ICAM-1 KO mice was incubated (4 hours at 37°C) with IL-1β (50 ng/mL), LTB4 (30 nM), KC (30 nM), fMLP (1 µM), LPS (300 ng/mL), or TNF (100 ng/mL). Fluorescent ZymTR particles (10 µg/mL) or DHR (1 µM) were added to samples for 15 minutes before RBC lysis and labeling with DAPI and fluorescent antibodies against CD45 and Ly6G. Samples were analyzed by flow cytometry. (A) Representative histogram illustrating the detection of ZymTR-associated neutrophils. (B) Percentage of ZymTRpos neutrophils in unstimulated and LPS-stimulated WT and ICAM-1 KO samples. (C) Percentage of DHR positive (DHRpos) neutrophils, in LPS-stimulated as compared with LPS-unstimulated controls was quantified in WT and ICAM-1 KO samples. (D) Frequency of DHR positive neutrophils within the ICAM-1 positive (pos) or negative (neg) populations in LPS-stimulated blood. Data are expressed as mean ± SEM of n = 4 to 24 animals/group. (E) Percentage of DHRpos neutrophils in WT neutrophils stimulated as shown. Statistically significant (t test) differences between treatment groups: **P < .01, ***P < .001; differences between WT and ICAM-1 KO: #P < .05, ###P < .001.
ICAM-1 expression facilitates enhanced neutrophil effector functions. Whole blood from WT or ICAM-1 KO mice was incubated (4 hours at 37°C) with IL-1β (50 ng/mL), LTB4 (30 nM), KC (30 nM), fMLP (1 µM), LPS (300 ng/mL), or TNF (100 ng/mL). Fluorescent ZymTR particles (10 µg/mL) or DHR (1 µM) were added to samples for 15 minutes before RBC lysis and labeling with DAPI and fluorescent antibodies against CD45 and Ly6G. Samples were analyzed by flow cytometry. (A) Representative histogram illustrating the detection of ZymTR-associated neutrophils. (B) Percentage of ZymTRpos neutrophils in unstimulated and LPS-stimulated WT and ICAM-1 KO samples. (C) Percentage of DHR positive (DHRpos) neutrophils, in LPS-stimulated as compared with LPS-unstimulated controls was quantified in WT and ICAM-1 KO samples. (D) Frequency of DHR positive neutrophils within the ICAM-1 positive (pos) or negative (neg) populations in LPS-stimulated blood. Data are expressed as mean ± SEM of n = 4 to 24 animals/group. (E) Percentage of DHRpos neutrophils in WT neutrophils stimulated as shown. Statistically significant (t test) differences between treatment groups: **P < .01, ***P < .001; differences between WT and ICAM-1 KO: #P < .05, ###P < .001.
Mechanism of neutrophil ICAM-1–mediated enhanced effector function?
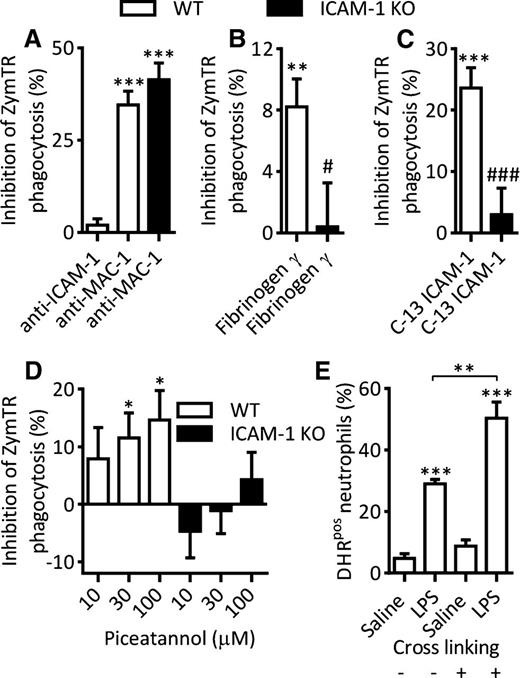
Having identified a role for neutrophil ICAM-1 in phagocytosis and ROS generation, the potential associated mechanisms were addressed. To test the hypothesis that cell-surface ICAM-1 may be involved in uptake of zymosan particles, the effect of an anti–ICAM-1 mAb that inhibits ICAM-1 integrin binding32,33 on LPS-induced phagocytosis was examined. In contrast to the effects seen under conditions of ICAM-1 genetic deletion (Figure 2B), surface blockade of ICAM-1 had no impact on zymosan phagocytosis (Figure 3A). Using the same protocol, as expected, an anti–Mac-1 mAb significantly inhibited LPS-induced zymosan phagocytosis with a similar level of suppression in both WT and ICAM-1 KO samples. Because no role for ICAM-1–integrin binding was seen, we hypothesized that neutrophil ICAM-1 may interact with the other important ICAM-1 ligand fibrinogen and hence support uptake of fibrinogen-coated zymosan particles. To test this possibility, we employed a peptide mimicking the γ domain of fibrinogen that competitively inhibits ICAM-1–fibrinogen interactions.34,35 Addition of this peptide to LPS-stimulated WT samples led to a small but significant inhibition of ZymTR phagocytosis (Figure 3B). Importantly, the peptide blocker had no effect on phagocytosis by LPS-stimulated ICAM-1 KO neutrophils (Figure 3B), indicating the specificity of the inhibitor for ICAM-1–mediated responses.
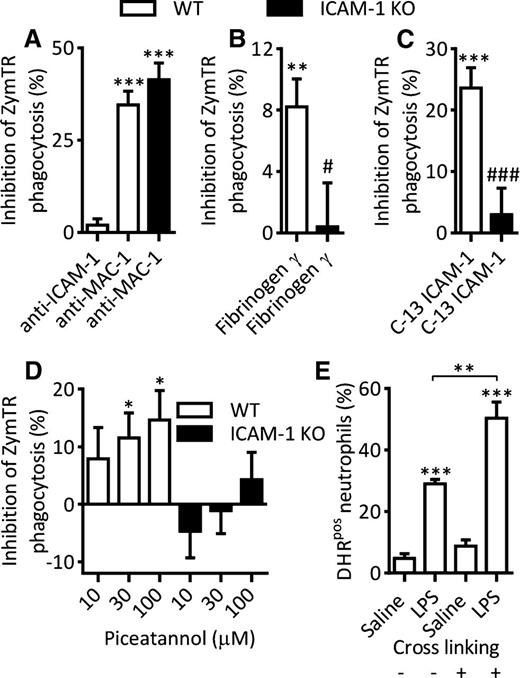
Mechanism of neutrophil ICAM-1–mediated enhanced effector functions. Whole blood from WT or ICAM-1 KO mice was incubated with LPS (300 ng/mL, 4 hours at 37°C). In some experiments, samples were treated with inhibitors as detailed before addition of ZymTR for a further 15 minutes. Unless indicated, results are presented as percentage inhibition of LPS-stimulated ZymTR phagocytosis. (A) LPS-stimulated samples were treated with blocking antibodies against ICAM-1 (clone YN1/1.7.4) or Mac-1 (clone M1/70) (both at 10 µg/mL for 15 minutes). (B) LPS-stimulated samples were treated with fibrinogen-γ–117-113 peptide to block ICAM-1–fibrinogen interaction (300 µM for 30 minutes). (C) LPS-stimulated samples were incubated with a membrane-penetrating peptide consisting of 13C-terminal amino acids of ICAM-1 that inhibits ICAM-1–mediated intracellular signaling (200 µg/mL for 120 minutes at 37°C). (D) LPS-stimulated WT or ICAM-1 KO samples were treated with the Syk inhibitor Piceatannol (30 minutes). (E) Percentage of DHRpos neutrophils in saline or LPS-stimulated samples following antibody crosslinking of ICAM-1 using rat–anti-ICAM-1 primary antibody (10 µg/mL, 15 minutes) and anti-rat secondary antibody (10 µg/mL, 15 minutes). Data are expressed as mean ± SEM of n = 8 to 15 animals/group. Statistically significant (t test) differences between control and treatment groups: *P < .05, **P < .01, and ***P < .001; differences between WT and ICAM-1 KO treatments: ###P < .001.
Mechanism of neutrophil ICAM-1–mediated enhanced effector functions. Whole blood from WT or ICAM-1 KO mice was incubated with LPS (300 ng/mL, 4 hours at 37°C). In some experiments, samples were treated with inhibitors as detailed before addition of ZymTR for a further 15 minutes. Unless indicated, results are presented as percentage inhibition of LPS-stimulated ZymTR phagocytosis. (A) LPS-stimulated samples were treated with blocking antibodies against ICAM-1 (clone YN1/1.7.4) or Mac-1 (clone M1/70) (both at 10 µg/mL for 15 minutes). (B) LPS-stimulated samples were treated with fibrinogen-γ–117-113 peptide to block ICAM-1–fibrinogen interaction (300 µM for 30 minutes). (C) LPS-stimulated samples were incubated with a membrane-penetrating peptide consisting of 13C-terminal amino acids of ICAM-1 that inhibits ICAM-1–mediated intracellular signaling (200 µg/mL for 120 minutes at 37°C). (D) LPS-stimulated WT or ICAM-1 KO samples were treated with the Syk inhibitor Piceatannol (30 minutes). (E) Percentage of DHRpos neutrophils in saline or LPS-stimulated samples following antibody crosslinking of ICAM-1 using rat–anti-ICAM-1 primary antibody (10 µg/mL, 15 minutes) and anti-rat secondary antibody (10 µg/mL, 15 minutes). Data are expressed as mean ± SEM of n = 8 to 15 animals/group. Statistically significant (t test) differences between control and treatment groups: *P < .05, **P < .01, and ***P < .001; differences between WT and ICAM-1 KO treatments: ###P < .001.
Because cell-surface binding capability of neutrophil ICAM-1 revealed a modest role in regulation of phagocytosis, we hypothesized that the principal role of neutrophil ICAM-1 in regulating neutrophil effector functions may reside in its signaling capability. To test this, the effect of a cell-permeant peptide that mimics the intracellular C-terminal domain of ICAM-1, widely used as a blocker of ICAM-1–mediated intracellular signaling,36,37 was investigated. Pretreatment of WT blood samples with the peptide led to significant inhibition of LPS-induced phagocytosis of zymosan particles (Figure 3C). Importantly, the peptide had no effect on phagocytosis responses detected in ICAM-1 KO samples (Figure 3C), indicating specificity of the peptide for ICAM-1–mediated events.
Because the cytoplasmic tyrosine kinase Syk has been linked to both ICAM-1 intracellular signaling38 and phagocytosis,39,40 the potential role of this kinase in LPS-induced phagocytosis was investigated using the inhibitor Piceatannol. Pretreatment of blood samples with Piceatannol dose-dependently inhibited LPS-induced ZymTR uptake in WT neutrophils. ICAM-1 KO neutrophils were less sensitive to Piceatannol, indicating that Syk-mediated mechanisms have an ICAM-1–dependent component (Figure 3D). Furthermore, antibody crosslinking of ICAM-1 that is known to activate intracellular signaling,10 enhanced LPS-stimulated ROS generation (Figure 3E), providing additional indication for the involvement of ICAM-1 signaling in regulation of neutrophil effector functions.
Collectively, these data provide evidence to suggest that ICAM-1 expression enhances neutrophil effector functions via interactions with fibrinogen and through intracellular signaling involving activation of Syk-mediated pathways.
ICAM-1 is expressed on neutrophils in models of endotoxemia in vivo
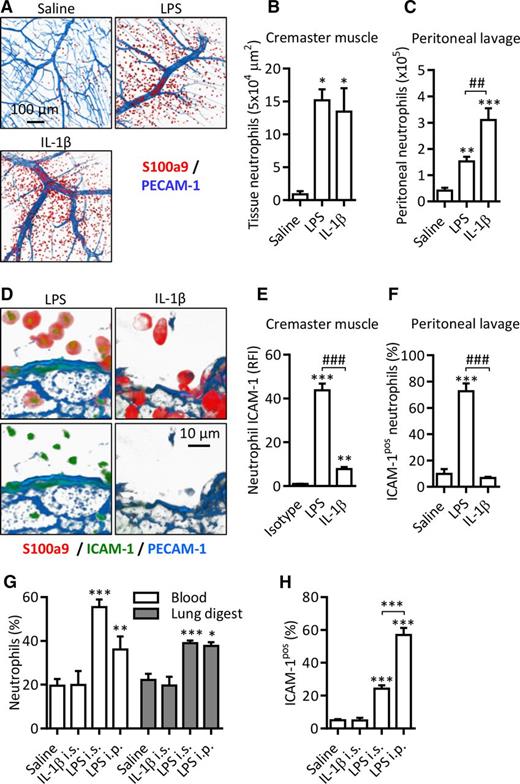
Having found that LPS induces surface expression of ICAM-1 on neutrophils and that this expression supports enhanced neutrophil effector functions in vitro, we next sought to investigate the expression and potential functions of neutrophil ICAM-1 in vivo. For this purpose, 2 murine models of local endotoxemia, namely LPS-stimulated cremaster muscles (IS) and LPS-induced peritonitis (IP), were employed. For comparison, the same models, as driven by locally injected IL-1β, a stimulus that did not induce neutrophil ICAM-1 expression in vitro (Figure 1A), were also investigated. Locally injected LPS and IL-1β induced significant neutrophil infiltration in the cremaster muscle and peritoneal cavity (Figure 4A-C). Furthermore, in both models, LPS-induced tissue-infiltrated neutrophils exhibited significant expression of ICAM-1 (Figure 4D-F). Of note, neutrophils accumulating in response to locally injected IL-1β showed very little or no ICAM-1 expression (Figure 4D-F). In both models, local LPS (but not IL-1β) appeared to exert a systemic effect in that there was a significant increase in the proportion of neutrophils in blood and in enzymatically digested lung tissues (Figure 4G). Importantly, IV administered fluorescently labeled anti-Ly6G antibody (used to label all intravascular neutrophils) before tissue collection indicated that in both naive and LPS-treated animals >90% of neutrophils in the lung digest were intravascular. As found with the tissue-infiltrated cells, in the LPS-driven reactions (but not IL-1β), an increase in the percentage of blood ICAM-1–positive neutrophils was detected (Figure 4H). Lung intravascular neutrophils also exhibited increased ICAM-1 expression following LPS administered IP (data not shown).
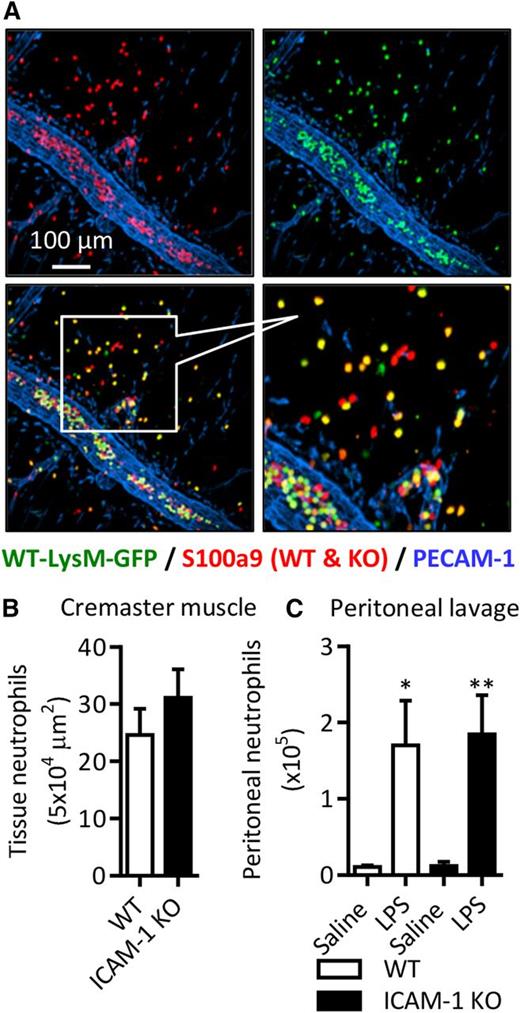
ICAM-1 is expressed on LPS-stimulated neutrophils in vivo. WT mice were injected with saline, LPS (300 ng IS or 1000 ng IP) or IL-1β (50 ng IP and IS). After a 4-hour in vivo test period, blood, lungs, peritoneal lavage, and cremaster muscles were collected. Cremaster muscles were fixed, permeabilized, and labeled with fluorescent antibodies against PECAM-1, the intracellular neutrophil marker S100a9, and ICAM-1 or an isotype control. Tissues were imaged by confocal microscopy and analyzed using Imaris. Following RBC lysis and enzymatic digestion of lung tissues, leukocytes from the blood, lung tissue digest, and peritoneal lavage were labeled with DAPI and fluorescent antibodies against CD45, Ly6G, ICAM-1, or an isotype control. (A) Representative images of unstimulated and stimulated cremaster muscles. (B-C) Quantification of extravasated neutrophils in saline and stimulated inflamed sites. (D) Representative images of ICAM-1 expression on extravasated neutrophils in LPS- and IL-β–stimulated cremaster muscles. (E-F) Quantification of ICAM-1 expression on extravasated neutrophils in inflamed tissues. (G) Percentage of Ly6G-positive neutrophils among CD45+ leukocytes in blood and digested lung tissue samples as quantified by flow cytometry. (H) Percentage of ICAM-1pos neutrophils in blood of saline, IL-1β–, and LPS-stimulated animals. Data are expressed as mean ± SEM of n = 3 to 10 animals/group. Statistically significant (t test or ANOVA) differences between stimulated and unstimulated treatment groups: *P < .05, **P < .01, and ***P < .001; differences between stimuli: ##P < .01 and ###P < .001.
ICAM-1 is expressed on LPS-stimulated neutrophils in vivo. WT mice were injected with saline, LPS (300 ng IS or 1000 ng IP) or IL-1β (50 ng IP and IS). After a 4-hour in vivo test period, blood, lungs, peritoneal lavage, and cremaster muscles were collected. Cremaster muscles were fixed, permeabilized, and labeled with fluorescent antibodies against PECAM-1, the intracellular neutrophil marker S100a9, and ICAM-1 or an isotype control. Tissues were imaged by confocal microscopy and analyzed using Imaris. Following RBC lysis and enzymatic digestion of lung tissues, leukocytes from the blood, lung tissue digest, and peritoneal lavage were labeled with DAPI and fluorescent antibodies against CD45, Ly6G, ICAM-1, or an isotype control. (A) Representative images of unstimulated and stimulated cremaster muscles. (B-C) Quantification of extravasated neutrophils in saline and stimulated inflamed sites. (D) Representative images of ICAM-1 expression on extravasated neutrophils in LPS- and IL-β–stimulated cremaster muscles. (E-F) Quantification of ICAM-1 expression on extravasated neutrophils in inflamed tissues. (G) Percentage of Ly6G-positive neutrophils among CD45+ leukocytes in blood and digested lung tissue samples as quantified by flow cytometry. (H) Percentage of ICAM-1pos neutrophils in blood of saline, IL-1β–, and LPS-stimulated animals. Data are expressed as mean ± SEM of n = 3 to 10 animals/group. Statistically significant (t test or ANOVA) differences between stimulated and unstimulated treatment groups: *P < .05, **P < .01, and ***P < .001; differences between stimuli: ##P < .01 and ###P < .001.
Collectively, these results demonstrate that vascular- and tissue-infiltrated neutrophils can express ICAM-1 on their cell surface in a stimulus-dependent manner.
Neutrophil ICAM-1 does not support neutrophil transmigration in vivo
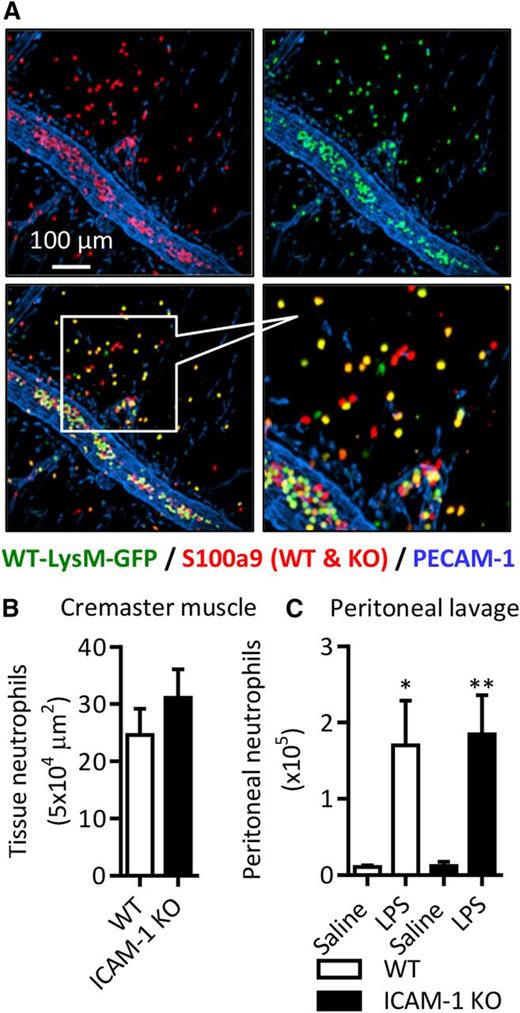
To investigate the functional role of neutrophil ICAM-1 in vivo, we generated chimeric mice expressing ICAM-1 on their vasculature but deficient in neutrophil ICAM-1. Lethally irradiated WT mice were reconstituted with a mixture of WT bone marrow hematopoietic cells from LysM-eGFP Ki mice (express GFP-labeled myeloid cells) and GFP-negative ICAM-1 KO mice. There was an average peripheral blood ratio of 60:40 WT:KO neutrophils in these animals (supplemental Figure 1A). This model provided a valuable tool for directly investigating the role of neutrophil ICAM-1 in neutrophil transmigration in vivo. In the cremaster muscle and peritonitis models, LPS-induced local neutrophil infiltration was quantified by confocal microscopy and flow cytometry, respectively, enabling WT and KO neutrophils to be easily distinguished in the same samples (ie, WT neutrophils were double positive for GFP and S100a9 or Ly6G, whereas ICAM-1 KO neutrophils were only S100a9 or Ly6G positive). With this rigorous approach, the same WT:KO ratio was seen in the peritoneal cavity as in the peripheral blood (supplemental Figure 1A). When the numbers of extravasated cells were normalized to the blood ratio of individual animals, no significant difference in tissue infiltration of WT or ICAM-1 KO neutrophils was noted (Figure 5A-C). These results demonstrate that neutrophil ICAM-1 is not required for acute neutrophil infiltration in response to local LPS.
Neutrophil ICAM-1 does not support neutrophil transmigration in vivo. Extravasation of WT or ICAM-1 KO neutrophils was quantified using chimeric WT mice with a mix of WT-LysM-GFP and ICAM-1 KO (GFP-negative) neutrophils. Chimeric mice were stimulated with LPS (1000 ng IS or IP) and cremaster muscle tissues and peritoneal lavage fluid was collected and labeled with fluorescent antibodies against PECAM-1 and S100a9 (cremasters) or CD45 and Ly6G (lavage). (A) Representative image of extravasated WT and ICAM-1 KO neutrophils in LPS-stimulated cremaster muscles. (B-C) Quantification of extravasated WT-LysM-GFP and ICAM-1 KO neutrophils in saline and/or LPS-stimulated inflamed tissues. Values are normalized to the WT:KO ratio in the peripheral blood of each animal. Data are expressed as mean ± SEM of n = 6 to 10 animals/group. Statistically significant (t test or ANOVA) differences between stimulated and unstimulated treatment groups: *P < .05 and **P < .01.
Neutrophil ICAM-1 does not support neutrophil transmigration in vivo. Extravasation of WT or ICAM-1 KO neutrophils was quantified using chimeric WT mice with a mix of WT-LysM-GFP and ICAM-1 KO (GFP-negative) neutrophils. Chimeric mice were stimulated with LPS (1000 ng IS or IP) and cremaster muscle tissues and peritoneal lavage fluid was collected and labeled with fluorescent antibodies against PECAM-1 and S100a9 (cremasters) or CD45 and Ly6G (lavage). (A) Representative image of extravasated WT and ICAM-1 KO neutrophils in LPS-stimulated cremaster muscles. (B-C) Quantification of extravasated WT-LysM-GFP and ICAM-1 KO neutrophils in saline and/or LPS-stimulated inflamed tissues. Values are normalized to the WT:KO ratio in the peripheral blood of each animal. Data are expressed as mean ± SEM of n = 6 to 10 animals/group. Statistically significant (t test or ANOVA) differences between stimulated and unstimulated treatment groups: *P < .05 and **P < .01.
ICAM-1 facilitates neutrophil phagocytosis in vivo
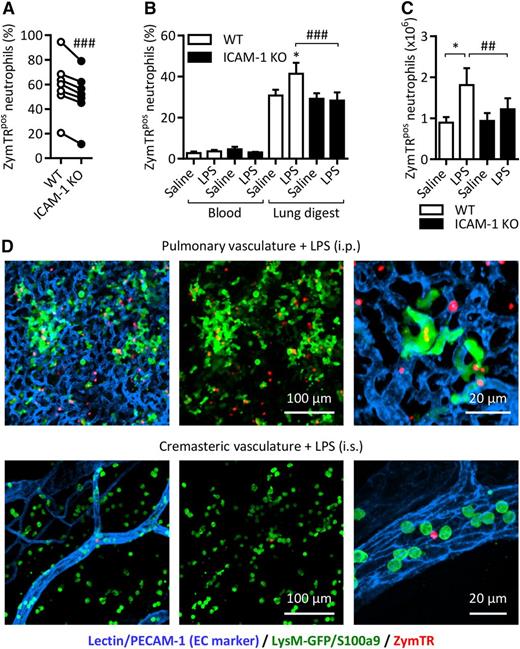
Because our in vitro studies identified phagocytosis as a principal effector function associated with neutrophil ICAM-1, we sought to investigate this association in vivo. For this purpose, we took advantage of the chimeric mice detailed previously. The mice were subjected to LPS-induced peritonitis, a model that induces blood neutrophilia (Figure 4G), tissue infiltration of neutrophils (Figure 4C) and ICAM-1 expression on intravascular and peritoneal neutrophils (Figure 4F,H). ZymTR was given IV or IP 15 minutes before sample and/or tissue collection and the percentage of ZymTRpos WT-LysM-eGFP and ICAM-1 KO neutrophils was quantified by flow cytometry. This technique enabled us to directly compare WT and ICAM-1 KO neutrophils in the same samples, overriding potential animal variations. With this approach, WT peritoneal neutrophils showed a robust phagocytosis response that was significantly reduced in ICAM-1 KO cells (Figure 6A).
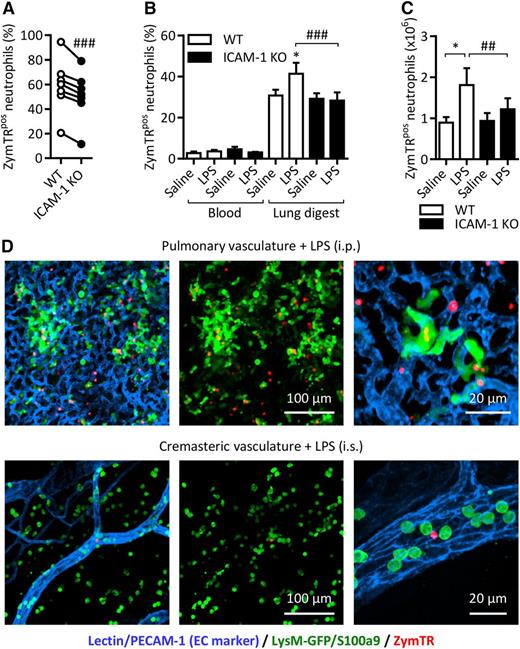
ICAM-1 facilitates neutrophil phagocytosis in vivo. Chimeric WT mice with a mix of WT-LysM-GFP and ICAM-1 KO (GFP-negative) neutrophils were injected with LPS (1000 ng IP). After 4 hours, ZymTR was injected IP (10 µg) or IV (100 µg) 15 minutes before collection of blood, lung tissues, and peritoneal lavage. Following RBC lysis and enzymatic digestion of lung tissues, cells were labeled with DAPI and fluorescent antibodies against CD45 and Ly6G and analyzed by flow cytometry. (A) Percentage of ZymTR-associated WT-LysM-GFP or ICAM-1 KO neutrophils in peritoneal lavage. (B) Percentage of ZymTR-associated WT-LysM-GFP or ICAM-1 KO neutrophils in blood and enzymatically digested lung tissues. (C) Total number of ZymTRpos WT-LysM-GFP or ICAM-1 KO neutrophils in the lungs of saline or LPS-stimulated animals. Values are normalized to the WT:KO ratio in the tissue of each animal. (D) Representative images showing neutrophils and ZymTR particles in LPS-stimulated pulmonary or cremasteric circulation 15 minutes after injection IV of ZymTR. Data are expressed as mean ± SEM of n = 6 to 10 animals/group. Statistically significant (t test) differences between treatment groups: *P < .05. Differences between WT-LysM-GFP and ICAM-1 KO: ##P < .01 and ###P < .001.
ICAM-1 facilitates neutrophil phagocytosis in vivo. Chimeric WT mice with a mix of WT-LysM-GFP and ICAM-1 KO (GFP-negative) neutrophils were injected with LPS (1000 ng IP). After 4 hours, ZymTR was injected IP (10 µg) or IV (100 µg) 15 minutes before collection of blood, lung tissues, and peritoneal lavage. Following RBC lysis and enzymatic digestion of lung tissues, cells were labeled with DAPI and fluorescent antibodies against CD45 and Ly6G and analyzed by flow cytometry. (A) Percentage of ZymTR-associated WT-LysM-GFP or ICAM-1 KO neutrophils in peritoneal lavage. (B) Percentage of ZymTR-associated WT-LysM-GFP or ICAM-1 KO neutrophils in blood and enzymatically digested lung tissues. (C) Total number of ZymTRpos WT-LysM-GFP or ICAM-1 KO neutrophils in the lungs of saline or LPS-stimulated animals. Values are normalized to the WT:KO ratio in the tissue of each animal. (D) Representative images showing neutrophils and ZymTR particles in LPS-stimulated pulmonary or cremasteric circulation 15 minutes after injection IV of ZymTR. Data are expressed as mean ± SEM of n = 6 to 10 animals/group. Statistically significant (t test) differences between treatment groups: *P < .05. Differences between WT-LysM-GFP and ICAM-1 KO: ##P < .01 and ###P < .001.
In contrast, blood neutrophils exhibited a low frequency of ZymTR uptake regardless of genotype or stimulation (Figure 6B). Interestingly, WT lung digest neutrophils, which represent predominantly pulmonary vascular neutrophils, showed a marked phagocytosis response in control animals that was further increased in LPS-stimulated mice (Figure 6B). This suggests that the slow blood flow of the pulmonary vasculature supports greater contact between circulating neutrophils and zymosan particles. Lung digest ICAM-1 KO neutrophils showed a similar level of ZymTRpos neutrophils in saline-treated mice, but no increase in phagocytosis was seen in LPS-stimulated ICAM-1 KO animals (Figure 6B). Analysis of these events in terms of the total number of ZymTRpos pulmonary vascular neutrophils showed a similar pattern. In LPS-stimulated mice, the total number of ZymTRpos WT neutrophils increased by 47.9% and in the same animals no such increase was seen with ICAM-1 KO cells (Figure 6C). Interestingly, there was a small but significant increase in the proportion of WT neutrophils in the lung tissue digest compared with blood, suggesting a potential preferential retention of WT cells in the lung vasculature (supplemental Figure 1). Confocal microscopy images of lung tissue of LPS-stimulated (IP) mice illustrate the dense network of small capillaries in lungs and also the notable presence of luminal ZymTRpos neutrophils (Figure 6D). In contrast, the microvessels of the cremaster are much less dense, and show little evidence of luminal ZymTR particles in LPS (IS)-stimulated tissues (Figure 6D).
Collectively, these results indicate that neutrophil ICAM-1 can support enhanced phagocytosis both within the vascular lumen (most notably in the pulmonary vasculature) and in the extravascular tissue.
Discussion
Despite the tremendous interest in the expression and function of endothelial cell ICAM-1 and the costimulatory role of ICAM-1 in lymphocytes, little is known about neutrophil-expressed ICAM-1. In the present study, we identify a stimulus-specific transcriptional regulation of ICAM-1 on murine neutrophils and report on the ability of certain stimuli to induce neutrophil ICAM-1 both in vitro and in vivo. Functionally, this expression was not essential for neutrophil migration into sites of inflammation, but supported enhanced neutrophil ROS generation and phagocytosis. The findings shed light on the mechanisms of expression of neutrophil ICAM-1 and identify it as a novel component of the host’s innate immune response to pathogens.
ICAM-1 is not commonly considered a neutrophil cell-surface antigen, but it has been detected on neutrophils in numerous infectious and inflammatory clinical settings.19,20,22 Because of the disparity of reports, a key objective of the present work was to gain a better understanding of the expression and regulation of expression of neutrophil ICAM-1, as studied using murine models. Initial in vitro experiments showed that in unstimulated murine blood, only ∼10% of neutrophils were ICAM-1–positive and that these cells expressed very low levels of the molecule (RFI < 2.0). Furthermore, stimulation with a wide range of neutrophil-activating and/or pro-inflammatory mediators (KC, fMLP, LTB4, and IL-1β) had no impact on neutrophil ICAM-1 expression. However LPS, TNF and zymosan particles induced a significant upregulation of ICAM-1 (∼60% to 80% ICAM-1–positive cells), indicating that this response is stimulus-specific. Of importance, LPS-induced upregulation of ICAM-1 was slow (∼3 hours) and was associated with the induction of ICAM-1 mRNA, suggesting that it is transcriptionally regulated. This finding is in line with transcriptional regulation of ICAM-1 expression in other cell types such as endothelial and epithelial cells, pericytes, and keratinocytes.4,7,9 Furthermore, it is now well-accepted that neutrophils are not transcriptionally static cells and in fact have the capability of significant de novo protein synthesis.41,42 Finally, our observed stimulus-specific pattern of murine neutrophil ICAM-1 expression is in agreement with reports that have linked human neutrophil ICAM-1 expression with the stimulants LPS and TNF,21,24,26 bacterial lipoprotein25 and S. aureus24 in vitro.
Because the profile of stimuli that induce neutrophil ICAM-1 are pathogen-derived and/or are commonly released under conditions of infection, we hypothesized that elevated neutrophil ICAM-1 may represent a physiological response associated with pathogen clearance. Indeed, although neutrophil ICAM-1 has to date been largely investigated in the context of neutrophil–neutrophil adhesion, aggregation,26,28 and neutrophil interactions with other components of the immune response,21 here we made the novel observation that neutrophil ICAM-1 supports an efficient phagocytosis response and is also associated with enhanced ROS generation. Although endothelial and epithelial cell ICAM-1 have been shown to support neutrophil phagocytosis by providing costimulatory signals through neutrophil integrins,43-45 to our knowledge this is the first report on the involvement of neutrophil ICAM-1 in neutrophil phagocytosis. Of note, ICAM-1 KO neutrophils did exhibit a significant increase in phagocytosis after LPS stimulation, indicating that, as expected, ICAM-1–independent phagocytic pathways such as those involving Mac-1/CR3 or FCγRs are also activated by LPS.46,47
Phagocytosis is a complex cellular process that is critical for innate immunity.46-49 It is an adhesion-dependent response, with the phagocytic vacuole or phagosome being formed following association of ligands on the surface of the particle (endogenous constituents of the target particle or serum-derived opsonins) with receptors on the cell surface of the phagocyte.46,48 Postbinding of the target, actin-driven intracellular events mediate particle engulfment.46,48 In addressing the mechanism through which neutrophil ICAM-1 mediates neutrophil phagocytosis, the functional role of the extracellular and intracellular domains of ICAM-1 was investigated. An antibody that blocks ICAM-1–integrin interactions had no effect on neutrophil phagocytosis of zymosan particles. However, because the blocking anti–ICAM-1 mAb employed has little or no inhibitory effect on ICAM-1 interactions with its other key ligand, fibrinogen,50-52 the potential involvement of neutrophil ICAM-1 binding to fibrinogen-coated zymosan particles was a possibility.2,34 The use of a competitive peptide antagonist of ICAM-1-fibrinogen interaction35,53 indicated a modest but significant and specific role for fibrinogen in ICAM-1–mediated neutrophil phagocytosis. Additionally, a membrane-penetrating peptide that mimics the intracellular domain of ICAM-1, and has been used to inhibit interactions of ICAM-1 with cytoplasmic accessory proteins,36,37 inhibited ICAM-1–dependent LPS-stimulated phagocytosis. Collectively, based on our results and published reports, we propose that cell-surface neutrophil ICAM-1 can mediate phagocytosis possibly independently of leukocyte integrins and possibly via interactions of neutrophil ICAM-1 with fibrinogen-coated particles. This interaction can then trigger ICAM-1–mediated signaling that supports efficient phagocytosis. The precise details of the latter are unclear and dissecting the different components was beyond the scope of the present study. However, because the cytoplasmic tyrosine kinase Syk has been linked to phagocytosis by neutrophils and macrophages39,40 and is activated by ICAM-1–mediated intracellular signaling in epithelial cells,38 the role of Syk in ICAM-1–mediated neutrophil phagocytosis was explored. Briefly, the Syk inhibitor Piceatannol induced a dose-dependent suppression of LPS-induced neutrophil phagocytosis, with the maximal effect being similar to that seen under conditions of ICAM-1 deletion. These results suggest a potential role for Syk in cell autonomous neutrophil ICAM-1–mediated phagocytosis. Of note, the majority of studies looking at ICAM-1 intracellular signaling have focused on endothelial ICAM-1 in the context of leukocyte transmigration.36,54,55 In such studies, clustering and dimerization of endothelial ICAM-1 via ligation of leukocyte integrins, or crosslinking with antibody-coated beads, promote association of the cytoplasmic domain with adaptor proteins and activation of subsequent effector pathways. These include modulation of actin polymerization, the formation of transmigratory cups that support leukocyte transmigration, and endocytosis of anti–ICAM-1 coated microbeads.6,13,56,57 Such events may also be involved in the mechanisms through which neutrophil ICAM-1 ligation supports particle engulfment.
Antibody crosslinking of neutrophil ICAM-1 also enhanced LPS-stimulated intracellular ROS generation. While it is attractive to speculate that this may be associated with pathogen killing within phagocytic vacuoles, it is also potentially possible that the ROS products generated support intracellular signaling. Indeed we observed high levels of ROS signal in cells that had engulfed zymosan (not shown), which may reflect activation of a “pathogen-killing” oxidative burst. In contrast, lower levels of ROS were noted in LPS-stimulated cells, a response that may reflect activation of signaling pathways.
ROS have been intimately associated with numerous signaling pathways triggered by ligation of endothelial cell adhesion molecules, including ICAM-1.58 Of relevance, ICAM-1 blockade inhibits production of ROS by neutrophils in response to granulocyte macrophage colony-stimulating factor or PMA,28 and ICAM-1 crosslinking has been linked to ROS generation and signaling in monocytes,59 indicating a broad role for myeloid cell ICAM-1 signaling as a regulator of superoxide anion generation. Finally, because neutrophils constitutively express the key ICAM-1 ligands, β2 integrins LFA-1 and Mac-1, ICAM-1–mediated neutrophil adhesion/aggregation may trigger ROS generation in an adhesion-dependent manner, as previously detailed.60
The expression and function of neutrophil ICAM-1 was also investigated in vivo using murine models of endotoxemia characterized by local neutrophil infiltration and blood neutrophilia. Within these models, tissue-infiltrated and blood vascular neutrophils exhibited enhanced ICAM-1 levels. Importantly, IL-1β–induced tissue transmigrated neutrophils showed no ICAM-1 expression indicating that: (1) In line with our in vitro findings, in vivo neutrophil ICAM-1 induction occurs in a stimulus-specific manner; and (2) neutrophil transmigration per se is not sufficient to elicit ICAM-1 expression on neutrophils. Of relevance, human neutrophils that have migrated through TNF-stimulated–cultured endothelial cells exhibit enhanced ICAM-1 expression, although the role of this remains unclear.27 Chimeric mice deficient in myeloid ICAM-1 indicated no involvement for neutrophil ICAM-1 in neutrophil tissue infiltration. In contrast to neutrophils, monocyte ICAM-1 has been shown to mediate adhesion and transendothelial migration, although the associated mechanism remains unknown.61
However, our chimeric mice did indicate a significant role for neutrophil ICAM-1 in phagocytosis of zymosan particles in vivo. This was evident with respect to both tissue-infiltrated and blood neutrophils, responses that could collectively reflect events involved in clearance of both tissue and bloodborne pathogens. With respect to the latter, IV injected zymosan particles were most effectively phagocytosed by neutrophils within the pulmonary vasculature. The pulmonary vasculature is composed of an extensive network of small-diameter alveolar capillaries through which leukocytes squeeze during normal recirculation. As a consequence, there is increased transit time of leukocytes through the lungs resulting in a large pool of slow-moving marginating leukocytes.62-65 This low-shear environment provides an ideal setting for bringing marginated neutrophils in close and prolonged proximity to bloodborne pathogens. Our data suggest that filtration of phagocytic targets from blood, as it passes through the pulmonary vasculature, may be enhanced by neutrophil-expressed ICAM-1. Hence, although it is well-accepted that lungs are an important host defense organ, this may involve pathogen clearance by phagocytes within the interstitial tissue as well as by circulating neutrophils within the pulmonary vasculature. Of relevance, although ICAM-1–deficient mice are protected from a number of sterile inflammatory conditions such as diabetic renal injury, radiation-induced lung injury, and atherosclerosis,66-68 they are highly susceptible to models of infections.69,70 These phenotypes are considered to be largely due to profound inhibition of leukocyte extravasation mediated by lack of endothelial cell ICAM-1. However, our results suggest that neutrophil ICAM-1 deficiency may also contribute to defective pathogen clearance and hence increased mortality in infectious models. In keeping with these findings is the observation that ICAM-1 KO neutrophils migrate equivalently to WT neutrophils into Streptococcus pneumoniae–infected lungs, but fail to control the pneumococcal infection.71 This hypothesis is in line with the fact that elevated neutrophil ICAM-1 has been reported in numerous infectious clinical setting involving bacterial and viral pathogens,19,20,72 further supporting the concept that elevated neutrophil ICAM-1 is a physiological component of innate immunity.
The mechanism through which neutrophils are retained in the pulmonary vasculature, most notably under conditions of infection, remains an elusive issue. Because fibrinogen can mediate leukocyte-endothelial adhesion,52 we propose that the interaction of neutrophil ICAM-1 with endothelial cell fibrinogen may provide a mechanism through which neutrophils accumulate in the pulmonary vascular compartment and contribute to luminal pathogen killing and clearance. In support of this, analysis of our chimeric mice indicated a higher ratio of WT:KO neutrophils in the pulmonary vasculature than in the rest of the circulation. Furthermore, fibrinogen-deficient mice exhibit delayed pulmonary injury in response to LPS,73 and although this has been linked to endothelial cell–associated fibrinogen binding to neutrophils via P-selectin and integrins, our data suggest that neutrophil ICAM-1 may also contribute to pulmonary retention.
In summary, our data provide insight into regulation of expression and function of neutrophil ICAM-1 in vitro and in vivo, most notably identifying it as a novel regulator of neutrophil phagocytosis. The findings highlight new avenues of research in host defense and suggest that induced expression of neutrophil ICAM-1 maybe a means through which pathogen clearance maybe enhanced in immunocompromised patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Ezra Aksoy for helpful discussions.
This work was supported by funds from the Wellcome Trust (098291/Z/12/Z and 101604/Z/13/Z) (S.N.) and the British Heart Foundation (FS/11/19/28761) (A.W.).
Authorship
Contribution: A.W. and M.B. designed and performed most experiments, analyzed data, and contributed to the writing of the manuscript; M.-B.V. designed and performed some immunofluorescent staining experiments; B.M. assisted with image acquisition and analysis; J.R.W. designed and performed reverse transcription- polymerase chain reaction experiments; P.L.H. contributed to the design of some of the experiments and the writing of the manuscript; N.H. provided valuable tools and contributed to the writing of the manuscript; and S.N. provided overall project supervision, contributed to the design of experiments and the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sussan Nourshargh, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: s.nourshargh@qmul.ac.uk.
References
Author notes
A.W. and M.B. contributed equally to this work.