In this issue of Blood, Hong et al advocate for use of additional US Food and Drug Administration (FDA)–approved safety measures for transfusion. Most patients transfused with contaminated platelets do not show immediate clinical signs. Active surveillance suggests patient risk 10- to 40-fold higher than passive hemovigilance.1
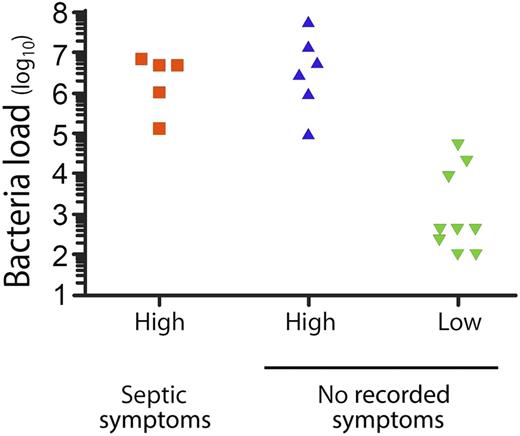
Correlation of high (>105) and low (<105) platelet bacterial load (in CFUs per mL) with documented overt symptoms of sepsis after transfusion. This figure has been adapted from Figure 1 in the article by Hong et al that begins on page 496.
Correlation of high (>105) and low (<105) platelet bacterial load (in CFUs per mL) with documented overt symptoms of sepsis after transfusion. This figure has been adapted from Figure 1 in the article by Hong et al that begins on page 496.
Platelet components contaminated with bacteria may cause acute septic reactions that are sometimes fatal. Despite optimal collection techniques, low numbers of bacteria commonly enter blood components at the time of phlebotomy, although the exact proportion of contaminated collections is not known.2 Platelets are stored at room temperature; however, many contaminating bacteria are obligate anaerobes and do not proliferate in the aerobic storage environment. Of those that do grow, 20% to 40% are detected by routine culture screening at blood centers, and the components are discarded.3 Nevertheless, culturing outdated platelets suggests that substantial numbers of bacterially contaminated components are transfused and that most cases either are not recognized by their physicians or are not reported to the blood bank under routine passive surveillance requirements. Consequently, septic reactions are reported for only ∼1:105 transfused platelets.2
Hong et al1 report their most recent 7-year experience with an ongoing study performed over the past ∼24 years at their institution. At the time of issue from the blood bank, a small aliquot of every platelet component is screened for bacteria using a relatively insensitive aerobic plate culture technique. The next day, any positive culture is investigated by clinical examination of the transfused patient and by medical record review. In this active surveillance process, 20 contaminated units were identified out of 51 440 screened (1:2572), mostly with gram-positive Staphylococcus species and Streptococcus species, organisms known to have caused severe reactions and fatalities in the past. Transfusion reactions were reported for none of these patients, yet 5 (1:10 720) had clinical signs and symptoms on retrospective review that were compatible with a septic reaction occurring 9 to 24 hours after transfusion, including 1 fatality. These reactions were all associated with components containing high concentrations of bacteria that were infused into neutropenic patients; 4 of 5 were transfused on day 5 of platelet storage and 1 on day 4 (Michael R. Jacobs, Case Western Reserve University School of Medicine, e-mail, December 2, 2015), and 4 of 5 were outpatient transfusions. Six other patients were transfused with platelet components containing similar high bacterial concentrations without a documented reaction, as were 9 patients transfused with lower bacterial concentrations (<105 colony-forming units [CFUs] per mL; see figure). Overall, 11 contaminated units were transfused on day 4 of storage (7 with concentrations <105 CFUs per mL), suggesting an additional increase in risk had the transfusion been delayed to day 5 (Michael R. Jacobs, Case Western Reserve University School of Medicine, e-mail, December 2, 2015). None of the recipients of contaminated platelets were on antibiotics at the time of transfusion. Concurrently, 284 transfusion reactions were reported in patients who received noncontaminated platelet components. Analysis of 6 sets of diagnostic criteria for septic transfusion reactions demonstrated their lack of sensitivity and specificity for detecting reactions to bacterially contaminated transfusions, most commonly because they failed to recognize delayed reactions. The authors conclude that bacterial sepsis continues to occur at high frequency especially in neutropenic patients, that passive hemovigilance fails to reliably detect such reactions, and that commonly used clinical diagnostic criteria are not useful for the recognition of transfusion reactions associated with exposure to bacterially contaminated platelets.
The experience of Hong et al is unique in that it is not routine practice to culture platelet products at the time of issue. It is, however, likely to be representative of the US situation, as they found similar rates of bacterial contamination in both apheresis and whole blood–derived platelets and from 2 different suppliers using the 2 available postcollection bacterial culture screening techniques. Their finding of a risk of sepsis of 1:10 720 and of bacterial exposure of 1:2572 platelet components is compounded in patients receiving multiple platelet transfusions, especially in highly immunocompromised allogeneic stem cell transplant patients, who may receive as many as 30 to 50 platelet transfusions and are particularly susceptible to infection. The frequency of septic reactions in outpatients is also of particular concern, as these may not be recognized and treated in a timely fashion. The clinical significance of transfusion of low concentrations of bacteria is unclear. Hong et al did not find evidence of positive blood cultures within 48 hours of transfusion; however, infused bacteria may sometimes seed to protected sites and cause delayed infections,4,5 and an effect on long-term outcome cannot be excluded.
In this setting, the FDA recommends the implementation of measures to improve the safety of platelet transfusions from bacterial contamination6 and has approved multiple novel technologies to that end, including 2 rapid diagnostic tests that are performed on the day of transfusion to detect high concentrations of bacteria (>105 CFUs per mL) and a pathogen reduction process that is performed soon after collection and is capable of inactivating most but not all bacteria, viruses, protozoa, and leukocytes. These recommendations have not been implemented by transfusion services because of cost and convenience considerations and the perception, reinforced by passive hemovigilance data, that bacterial contamination is a remote risk. The data presented here render the latter view untenable. Snyder et al previously proposed that the FDA mandate the use of these approved technologies to reduce the risk of bacterial contamination, as a means to ensure universal implementation.7 In the interim, individual institutions and physicians should decide what is best for their patients and insist that they are appropriately protected using available FDA-approved technologies, as recommended by Hong et al.
Conflict-of-interest disclosure: Subsequent to the first review of this manuscript, R.J.B. took on the role of Chief Medical Officer at Cerus Corporation (Concord, CA).