Key Points
Human Lin− CD34hi CD117int/hi FcεRI+ cells in blood constitute mast cell progenitors.
Asthmatics with reduced lung function have an increased frequency of circulating mast cell progenitors.
Abstract
Mast cells are rare tissue-resident immune cells that are involved in allergic reactions, and their numbers are increased in the lungs of asthmatics. Murine lung mast cells arise from committed bone marrow–derived progenitors that enter the blood circulation, migrate through the pulmonary endothelium, and mature in the tissue. In humans, mast cells can be cultured from multipotent CD34+ progenitor cells. However, a population of distinct precursor cells that give rise to mast cells has remained undiscovered. To our knowledge, this is the first report of human lineage-negative (Lin−) CD34hi CD117int/hi FcεRI+ progenitor cells, which represented only 0.0053% of the isolated blood cells in healthy individuals. These cells expressed integrin β7 and developed a mast cell–like phenotype, although with a slow cell division capacity in vitro. Isolated Lin− CD34hi CD117int/hi FcεRI+ blood cells had an immature mast cell–like appearance and expressed high levels of many mast cell–related genes as compared with human blood basophils in whole-transcriptome microarray analyses. Furthermore, serglycin, tryptase, and carboxypeptidase A messenger RNA transcripts were detected by quantitative reverse transcription–polymerase chain reaction. Altogether, we propose that the Lin− CD34hi CD117int/hi FcεRI+ blood cells are closely related to human tissue mast cells and likely constitute an immediate precursor population, which can give rise to predominantly mast cells. Furthermore, asthmatics with reduced lung function had a higher frequency of Lin− CD34hi CD117int/hi FcεRI+ blood mast cell progenitors than asthmatics with normal lung function.
Introduction
Mast cells are infamous for their role in allergic disease, and their activation can lead to a severe life-threatening condition, an anaphylactic reaction.1 Most recognized is the powerful mast cell activation caused by allergen cross-linking of immunoglobulin E–loaded high-affinity immunoglobulin E receptors (FcεRIs), which leads to the release of an array of different mediators.2 In allergic asthmatics, mast cell mediators such as histamine and prostaglandin D2 are secreted rapidly after allergen provocation.3-5 These mediators are devastating to the asthmatic lung causing, for example, bronchoconstriction.6,7 In comparison with healthy individuals, the mast cell numbers are increased in the airway smooth muscle8 and alveolar parenchyma9 of asthmatics. As a consequence, a high number of mast cells can be activated during allergen exposure, and the symptoms can be severe.
Mast cells originate from the bone marrow but are absent in the blood in their fully granulated mature state. In mice, mast cell progenitors are present in the circulation and mature on arrival in the peripheral tissues.10 Progenitors committed to the mast cell lineage can be found in the bone marrow11,12 and circulate in the blood of naïve mice at very low frequencies as lineage-negative (Lin−) c-kithi (CD117hi) ST2+ integrin β7hi CD16/32hi FcεRI+ or FcεRI− cells.13 Virtually all mouse mast cell progenitors express FcεRI once entering peripheral tissues, such as the lungs and the peritoneal cavity.14 In mice with experimental allergic asthma, mast cell progenitors are recruited to the lung15 and give rise to increased numbers of lung mast cells.16-18 In humans, mast cells can be derived from CD34+19,20 and CD34+ CD117+21 progenitor cells in peripheral blood by in vitro culture. However, whether human mast cells originate from a distinct population of progenitors has not previously been determined. Identification of the ancestor of mast cells is important for understanding the underlying mechanisms of allergic disorders and hematologic diseases such as systemic mastocytosis. Possibly, such progenitors would be a novel drug target in mast cell–related diseases.
Because FcεRI is involved in allergen-induced mast cell activation in asthma, the goal of the present investigation was to identify novel human blood mononuclear cell populations that could give rise to CD117+ FcεRI+ mast cells. In vitro culture of prospectively isolated CD34+ blood progenitors showed that the CD117+ FcεRI+ mast cell–forming potential was mainly found in the Lin− CD34hi CD117int/hi FcεRI+ cell fraction. This population of blood cells contained high levels of mast cell–associated genes in comparison with human blood basophils and had detectable levels of messenger RNA (mRNA) transcripts of, for example, tryptase. Collectively, the data suggest that this rare population of blood cells constitutes precursors to human tissue mast cells.
Methods
Blood samples
Blood samples were obtained from 13 patients with allergic asthma (median age 24 years, range 16-36 years; 9 women; median asthma control test22 21, range 17-25), 1 patient with nonallergic asthma (age 14 years, male, asthma control test 21), and 10 healthy, nonallergic controls (median age 20 years, range 16-35 years; 7 women) who participated in the follow-up of the MIDAS (Minimally-Invasive Diagnostic Procedures in Allergy, Asthma or Food Hypersensitivity) study.23-25 The MIDAS and the follow-up MIDAS II study were performed in 2010-2012 and 2013-2015, respectively. Two of the subjects with allergic asthma did not have anti-inflammatory treatment (inhaled corticosteroids or antileukotrienes) the year previous to this study, and 4 of the subjects with allergic asthma received only short treatment periods with anti-inflammatory treatment during the past 3 months. The rest of the asthmatic patients reported daily anti-inflammatory treatment during the past 3 months previous to the MIDAS II study. The prebronchodilator forced expiratory volume in 1 second (FEV1) was recorded in all subjects in connection to blood sampling and subsequent mast cell progenitor analysis. The results were expressed as percent of the predicted value.25
Lung tissue
Lung tissues were obtained from 2 patients undergoing lung resection because of lung cancer. The tissue samples were taken from a tumor-free part of the resected lobe.
Cell extraction
Mononuclear cells from blood were enriched from EDTA-treated whole blood using Ficoll-Paque Premium (ρ = 1.077 g/mL; GE Healthcare, Little Chalfont, UK). Platelets were removed by centrifugation. Human lung tissues were processed using the Human Tumor Dissociation Kit, gentleMACS C tubes, and the gentleMACS Octo Dissociator with Heaters (all from Miltenyi Biotec, Bergisch Gladbach, Germany). The 37C_m_LDK_1 program was used. Red blood cells were lysed using an ammonium chloride potassium buffer (0.15 M NH4Cl, 10 mM KHCO3, and 1 mM EDTA, pH 7.4). The cells were counted using a hemocytometer.
Flow cytometry and cell sorting
The following fluorochrome-conjugated antibodies were used: CD4 (RPA-T4), CD8 (RPA-T8), CD13 (WM15), CD14 (M5E2), CD19 (HIB19), CD34 (581), CD45RA (HI100), CD117 (104D2), CRTH2 (BM16), FcεRI (AER-37), and integrin β7 (FIB504) (all from BD Biosciences, Franklin Lakes, NJ, and eBiosciences, San Diego, CA). Staining of cells was performed in phosphate-buffered saline pH 7.4 with 2% heat-inactivated fetal calf serum. Flow cytometry and cell sorting were performed on an LSR II or a FACS Aria III (BD Biosciences). Internal controls were used to set the gates. Cells were sorted into 96-well plates or 60-well Terasaki plates.
Cell culture
Cells were cultured in serum-free Stempro-34 SFM medium (ThermoFisher, Waltham, MA) with 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine (Sigma-Aldrich). The medium was supplemented with 30 ng/mL interleukin (IL) 3, 100 ng/mL IL-6, 20 ng/mL IL-9, and 100 ng/mL stem cell factor alone or with addition of 20 ng/mL IL-5, 50 ng/mL granulocyte macrophage–colony-stimulating factor, 20 ng/mL thrombopoietin, 10 ng/mL IL-11, 10 ng/mL FMS-like tyrosine kinase 3 ligand, and 4 U/mL erythropoietin. Cytokines were from PeproTech (Rocky Hill, NJ) and R&D Systems (Minneapolis, MN). HMC-1 cells26 were cultured as described.27
Cell visualization
Tryptase activity in cytocentrifuged cells was detected with Z-Gly-Pro-Arg-4-methoxy-2-naphthylamide peptide substrate (MP Biomedicals, Santa Ana, CA).28 Standard protocols were followed for May-Grünwald Giemsa staining. Shandon Octospot was used to cytocentrifuge small number of cells (ThermoFisher). Images were captured as described.14
Microarray analysis and qRT-PCR
Ninety-one to 100 cells of each population were sorted into separate Terasaki plate wells containing 12 μL direct lysis buffer, provided with the Ovation One-Direct System (Nugen Technologies Inc., San Carlos, CA). Whole-transcriptome analyses were performed as described previously,14 with the exception that GeneChip Human Gene 2.0 ST Arrays were used. The raw data were normalized using the robust multiarray method.29,30 The gene expression data have been deposited in National Center for Biotechnology Information’s Gene Expression Omnibus database (accession number GSE69030; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE69030). A gene set enrichment analysis (GSEA) was performed as described previously.31,32 Gene sets <15 and >200 were filtered out. To establish the rank lists, default settings were used. Processes with an enrichment score >0.5 with a false discovery rate q value <0.05 were considered active. For quantitative reverse transcription–polymerase chain reaction (qRT-PCR), RNA was isolated from lysates of Lin− CD34hi CD117int/hi FcεRI+ cells and HMC-1 cells using NucleoSpin RNA II (Macherey-Nagel, Dueren, Germany), and first-strand complementary DNA (cDNA) was synthesized using total RNA as template by iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Gene expression levels were determined using the primer pairs in Table 1. The primers for the proteases were previously published.20 qRT-PCR was performed using SYBR GreenER SuperMix (ThermoFisher), 200 nM primers, following recommended PCR cycling conditions. Melting curve analysis was performed to ensure product uniformity.
Primers used for qRT-PCR
| Target gene . | Forward sequence (5′-3′) . | Reverse sequence (5′-3′) . |
|---|---|---|
| Actb | CGC GAG AAG ATG ACC CAG AT | GAT GGG CAC AGT GTG GGT G |
| Cma1 | TGC TCA TTG TGC AGG AAG GTC | ACC TCA AGC TTC TGC CAT GTG |
| Cpa3 | CCA GAT GCT ATT GTT TCC CTA TGG | GTA GCG GGT TTC ATA TCG AGT TG |
| Gapdh | CCA CAT CGC TCA GAC ACC AT | GGC AAC AAT ATC CAC TTT ACC AGA G |
| Gzmb | GGA AGA TCG AAA GTG CGA ATC | CTG GGC CAC CTT GTT ACA CA |
| Srgn | TGG GAG TGG CTT CCT AAC GGA | TGT CTG AGG GCA GAT TCC TGT |
| Tpsab1/Tpsb2 | CGA TGT GGA CAA TGA TGA GC | CGC CAA GGT GGT ATT TTG C |
| Target gene . | Forward sequence (5′-3′) . | Reverse sequence (5′-3′) . |
|---|---|---|
| Actb | CGC GAG AAG ATG ACC CAG AT | GAT GGG CAC AGT GTG GGT G |
| Cma1 | TGC TCA TTG TGC AGG AAG GTC | ACC TCA AGC TTC TGC CAT GTG |
| Cpa3 | CCA GAT GCT ATT GTT TCC CTA TGG | GTA GCG GGT TTC ATA TCG AGT TG |
| Gapdh | CCA CAT CGC TCA GAC ACC AT | GGC AAC AAT ATC CAC TTT ACC AGA G |
| Gzmb | GGA AGA TCG AAA GTG CGA ATC | CTG GGC CAC CTT GTT ACA CA |
| Srgn | TGG GAG TGG CTT CCT AAC GGA | TGT CTG AGG GCA GAT TCC TGT |
| Tpsab1/Tpsb2 | CGA TGT GGA CAA TGA TGA GC | CGC CAA GGT GGT ATT TTG C |
Ethics
Informed written consent was obtained from all participants, and the study was approved by Uppsala Regional Ethical Review Board.
Statistical analysis
The statistical tests used are indicated in the respective figure legends. A P value <.05 was considered significant.
Results
Lin− CD34hi CD117int/hi FcεRI+ blood cells constitute mast cell progenitors
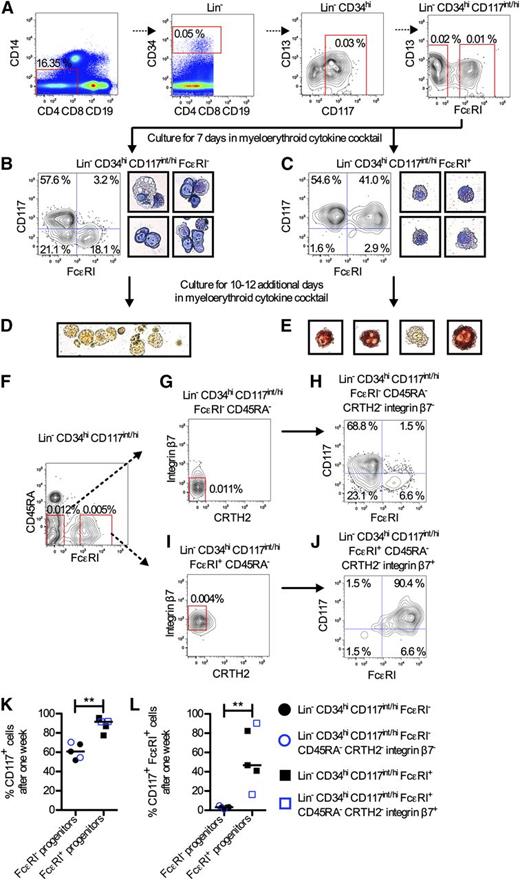
Progenitors with mast cell–forming potential have been found in human peripheral blood. These cells were enriched in the CD34+ CD117+ CD13+ fraction.21 In the present investigation, subpopulations of the CD34+ CD117+ CD13+ cells were sorted to identify committed mast cell progenitors. To isolate rare progenitor cell populations, the Lin markers CD4, CD8, CD19, and CD14 were included in the analysis. Lin− CD34hi CD117int/hi CD13+ cells were divided and sorted into FcεRI− and FcεRI+ cells. After 1 week in culture with IL-3, IL-6, IL-9, and stem cell factor, the Lin− CD34hi CD117int/hi CD13+ FcεRI− cells had formed only 4.4% CD117+ FcεRI+ mast cells (supplemental Figure 1, available on the Blood Web site). In contrast, Lin− CD34hi CD117int/hi CD13+ FcεRI+ cells had formed 75.3% CD117+ FcεRI+ mast cells (supplemental Figure 1). FcεRI+ cells were also found in the Lin− CD34hi CD117int/hi CD13−/lo fraction (supplemental Figure 2). Therefore, the mast cell potential was evaluated in the total Lin− CD34hi CD117int/hi FcεRI+ fraction irrespective of CD13 expression (Figure 1A). Lin− CD34hi CD117int/hi FcεRI+ cells had formed 72.9% and 78.8% CD117+ FcεRI+ mast cells after 2 and 7 days, respectively (supplemental Figure 3). Lin− CD34hi CD117int/hi FcεRI− cells were used as control. These cells had only formed 2.7% and 5.7% CD117+ FcεRI+ mast cells, respectively (supplemental Figure 3). As the mast cell–forming capacity was high among the Lin− CD34hi CD117int/hi FcεRI+ cells, their level of commitment was evaluated. Lin− CD34hi CD117int/hi FcεRI+ cells were cultured with a myeloerythroid cytokine cocktail (Figure 1A). Lin− CD34hi CD117int/hi FcεRI− cells were cultured as control. The sorted cells were analyzed already after 7 days to enable analysis of short-lived cells that could be present in the culture. The Lin− CD34hi CD117int/hi FcεRI+ cells mainly formed CD117+ cells, which contained granules and had a mast cell–like morphology (Figure 1C). Approximately half of the cells were CD117+ FcεRI+ mast cells (Figure 1C,K-L). In contrast, the Lin− CD34hi CD117int/hi FcεRI− cell fraction formed heterogeneous cells, a lower frequency of CD117+ cells, and almost no CD117+ FcεRI+ mast cells after 1 week (Figure 1B,K-L). A distinct population of CD117− FcεRI+ cells was also developed. Thus, the blood progenitors within the Lin− CD34hi CD117int/hi FcεRI− population likely constitute multipotent progenitors. Some dead cells without stainable nucleus and structures in the cytoplasm were observed in cells cultured for 7 days, both from the Lin− CD34hi CD117int/hi FcεRI− and the Lin− CD34hi CD117int/hi FcεRI+ cell fraction (supplemental Figure 4).
Primary Lin− CD34hi CD117int/hi FcεRI+ blood cells constitute mast cell progenitors. (A) Enriched mononuclear cells from blood were analyzed with flow cytometry. (B-C) Lin− CD34hi CD117int/hi FcεRI− and Lin− CD34hi CD117int/hi FcεRI+ cells were cultured in a myeloerythroid cytokine cocktail containing IL-3, IL-5, IL-6, IL-9, IL-11, FMS-like tyrosine kinase 3 ligand, stem cell factor, thrombopoietin, erythropoietin, and granulocyte macrophage–colony-stimulating factor for 7 days and analyzed with flow cytometry and May-Grünwald Giemsa staining. The results in panels B and C are representative of 3 independent experiments. The width of each photo corresponds to 50 μm. (D-E) Lin− CD34hi CD117int/hi FcεRI− and Lin− CD34hi CD117int/hi FcεRI+ cells were cultured for a total of 17 to 19 days in a myeloerythroid cytokine cocktail, and enzyme histochemical staining of tryptase activity was performed to identify tryptase-containing cells. Red/brown color indicates positive staining. The results in panels D and E are representative of 3 independent experiments. The width of the photo in panel D corresponds to 160 μm, whereas the width of each photo in panel E corresponds to 40 μm. (F-G,I) Lin− CD34hi CD117int/hi cells were analyzed with flow cytometry. Lin− CD34hi CD117int/hi FcεRI− CD45RA− CRTH2− integrin β7− (H) and Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ (J) cells were sorted and cultured in a myeloerythroid cytokine cocktail for 7 days and analyzed with flow cytometry. The results in panels H and J are from 1 out of 2 independent experiments. The frequency of CD117+ cells (K) or CD117+ FcεRI+ cells (L) was analyzed after culturing subpopulations of Lin− CD34hi CD117int/hi blood progenitors in a myeloerythroid cytokine cocktail for 7 days. The median values are shown. The differences between groups were evaluated using the 2-tailed Mann-Whitney U test. **P < .01.
Primary Lin− CD34hi CD117int/hi FcεRI+ blood cells constitute mast cell progenitors. (A) Enriched mononuclear cells from blood were analyzed with flow cytometry. (B-C) Lin− CD34hi CD117int/hi FcεRI− and Lin− CD34hi CD117int/hi FcεRI+ cells were cultured in a myeloerythroid cytokine cocktail containing IL-3, IL-5, IL-6, IL-9, IL-11, FMS-like tyrosine kinase 3 ligand, stem cell factor, thrombopoietin, erythropoietin, and granulocyte macrophage–colony-stimulating factor for 7 days and analyzed with flow cytometry and May-Grünwald Giemsa staining. The results in panels B and C are representative of 3 independent experiments. The width of each photo corresponds to 50 μm. (D-E) Lin− CD34hi CD117int/hi FcεRI− and Lin− CD34hi CD117int/hi FcεRI+ cells were cultured for a total of 17 to 19 days in a myeloerythroid cytokine cocktail, and enzyme histochemical staining of tryptase activity was performed to identify tryptase-containing cells. Red/brown color indicates positive staining. The results in panels D and E are representative of 3 independent experiments. The width of the photo in panel D corresponds to 160 μm, whereas the width of each photo in panel E corresponds to 40 μm. (F-G,I) Lin− CD34hi CD117int/hi cells were analyzed with flow cytometry. Lin− CD34hi CD117int/hi FcεRI− CD45RA− CRTH2− integrin β7− (H) and Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ (J) cells were sorted and cultured in a myeloerythroid cytokine cocktail for 7 days and analyzed with flow cytometry. The results in panels H and J are from 1 out of 2 independent experiments. The frequency of CD117+ cells (K) or CD117+ FcεRI+ cells (L) was analyzed after culturing subpopulations of Lin− CD34hi CD117int/hi blood progenitors in a myeloerythroid cytokine cocktail for 7 days. The median values are shown. The differences between groups were evaluated using the 2-tailed Mann-Whitney U test. **P < .01.
Depending on culture conditions mast cells derived from peripheral blood cells contain tryptase to a high degree after several weeks in culture.20,33 To confirm that the Lin− CD34hi CD117int/hi FcεRI+ progenitors could mature in vitro, cell-associated trypsin-like activity was used as a measure of mast cell tryptase content. Already after 17 to 19 days of culture in the myeloerythroid cytokine cocktail, a fraction of the Lin− CD34hi CD117int/hi FcεRI+ cells had tryptase activity, indicating that these cells had matured (Figure 1E). As expected, the Lin− CD34hi CD117int/hi FcεRI− cells were overall negative for tryptase activity after 17 to 19 days of culture (Figure 1D). However, occasional cells stained positive for tryptase activity (supplemental Figure 5).
Three additional markers were added to the antibody panel in an attempt to enrich the mast cell potential within the Lin− CD34hi CD117int/hi FcεRI+ fraction. CD45RA, which was absent on virtually all of the Lin− CD34hi CD117int/hi FcεRI+ cells, was used to exclude granulocyte/monocyte as well as lymphoid progenitors (Figure 1F). Integrin β7, which is expressed by mouse mast cell progenitors, was found on the majority of the Lin− CD34hi CD117int/hi CD45RA− FcεRI+ cells (Figure 1I). CRTH2, which is expressed on mature basophils and mast cells, was absent on essentially all of the Lin− CD34hi CD117int/hi FcεRI+ progenitors (Figure 1I). Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ cells were sorted and cultured with the myeloerythroid cytokine cocktail (Figure 1J). Lin− CD34hi CD117int/hi FcεRI− CD45RA− CRTH2− integrin β7− cells were used as control (Figure 1H). The frequencies of CD117+ cells and CD117+ FcεRI+ mast cells were higher in the Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ fraction than in the Lin− CD34hi CD117int/hi FcεRI− CD45RA− CRTH2− integrin β7− fraction after 1 week in culture (Figure 1H,J-L). In 1 experiment, the CD117+ FcεRI+ mast cells even reached 90.4% after 1 week (Figure 1J,L). However, in a second experiment, 16% of the progeny constituted CD117+ FcεRI+ mast cells (Figure 1L), which revealed a large variation in frequency of CD117+ FcεRI+ mast cells after in vitro culture between individuals. Even though only 16% of the Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ progeny constituted CD117+ FcεRI+ mast cells in this second subject, 92% were still CD117+ cells (Figure 1K). We concluded that even though the Lin− CD34hi CD117int/hi FcεRI+ mast cell progenitors could be classified as CD45RA− integrin β7+ CRTH2− cells, these 3 markers did not enhance the frequency of CD117+ cells retaining FcεRI expression 7 days postculture of the mast cell progenitors (Figure 1L). In conclusion, the Lin− CD34hi CD117int/hi FcεRI+ characteristics alone are sufficient to identify this rare blood cell population.
Lin− CD34hi CD117int/hi FcεRI+ and Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ mast cell progenitors are small, slowly dividing cells that become granulated upon culture
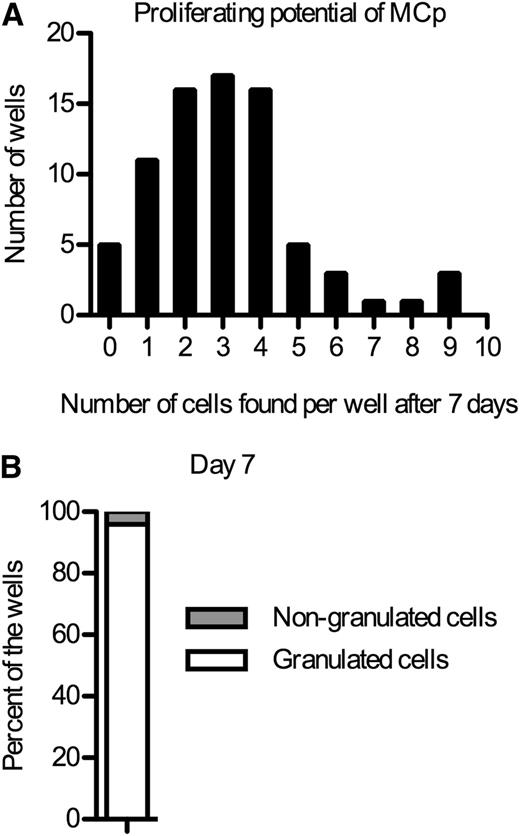
To check if single Lin− CD34hi CD117int/hi FcεRI+ or Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ progenitors could give rise to different cell lineages, single cells were sorted. After 7 days, the number of cells that each single progenitor gave rise to was evaluated (Figure 2A). The median was 3 cells after 1 week in culture, indicating that the progenitors divide slowly. May-Grünwald staining of the cytocentrifuged cells indicated that the vast majority of the cells had developed granules, some of which stained metachromatically. In fact, 24 out of 25 individual Lin− CD34hi CD117int/hi FcεRI+ or Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ progenitors had exclusively given rise to granulated cells (Figure 2B). From a single well, 1 cell that lacked granules was recovered. No other cells were recovered from the same well, and no well containing both nongranulated and granulated cells was identified. However, because of the slow division of the single cells, it is not possible to draw definite conclusions whether one progenitor could give rise to some other cell type besides mast cells.
Blood mast cell progenitors divide slowly. (A) Single Lin− CD34hi CD117int/hi FcεRI+ or Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ mast cell progenitors (MCp) were sorted into individual wells and cultured in a myeloerythroid cytokine cocktail. The number of cells in each well was counted after 7 days. (B) Cytocentrifuged and May-Grünwald Giemsa–stained cells from the wells in panel A were characterized based on morphology. At least 1 cell was identified from 25 of the wells in panel A. The Lin− CD34hi CD117int/hi FcεRI+ and the Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ cells were sorted in 1 experiment each, and the results from each experiment were pooled.
Blood mast cell progenitors divide slowly. (A) Single Lin− CD34hi CD117int/hi FcεRI+ or Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ mast cell progenitors (MCp) were sorted into individual wells and cultured in a myeloerythroid cytokine cocktail. The number of cells in each well was counted after 7 days. (B) Cytocentrifuged and May-Grünwald Giemsa–stained cells from the wells in panel A were characterized based on morphology. At least 1 cell was identified from 25 of the wells in panel A. The Lin− CD34hi CD117int/hi FcεRI+ and the Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ cells were sorted in 1 experiment each, and the results from each experiment were pooled.
Next, Lin− CD34hi CD117int/hi FcεRI+ and Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ progenitors were sorted and stained with May-Grünwald Giemsa without culturing the cells. These progenitors were small. Some of the cells lacked granules, whereas some had few metachromatic granules (Figure 3A). To provide a morphologic comparison with the blood mast cell progenitors, Lin− CD117+ FcεRI+ human lung mast cells and Lin− CRTH2+ FcεRI+ blood basophils were sorted and stained with May-Grünwald Giemsa. In contrast to the mast cell progenitors, Lin− CD117+ FcεRI+ mature lung mast cells and Lin− CRTH2+ FcεRI+ mature blood basophils contained numerous metachromatic granules (Figure 3B-C). The lung mast cells and the blood basophils had no or low expression of CD34, which indicates that they were mature (Figure 3D-E). In summary, Lin− CD34hi CD117int/hi FcεRI+ and Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ progenitors have an immature morphology, but they are in the process of forming granules.
Primary blood mast cell progenitors have no or few granules. (A) Lin− CD34hi CD117int/hi FcεRI+ cells or Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ mast cell progenitors (MCp) were sorted and stained with May-Grünwald Giemsa. (B) Sorted mature lung mast cells stained with May-Grünwald Giemsa. (C) Sorted mature blood basophils stained with May-Grünwald Giemsa. The width of each photo in panels A-C corresponds to 40 μm. (D) Gating strategy for identifying mature lung mast cells. (E) Gating strategy for identifying mature blood basophils. Mature lung mast cells and mature blood basophils were sorted in 2 independent experiments. The photos of blood mast cell progenitors were representative of cells from 4 independent experiments, where either Lin− CD34hi CD117int/hi FcεRI+ or Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ cells were isolated.
Primary blood mast cell progenitors have no or few granules. (A) Lin− CD34hi CD117int/hi FcεRI+ cells or Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ mast cell progenitors (MCp) were sorted and stained with May-Grünwald Giemsa. (B) Sorted mature lung mast cells stained with May-Grünwald Giemsa. (C) Sorted mature blood basophils stained with May-Grünwald Giemsa. The width of each photo in panels A-C corresponds to 40 μm. (D) Gating strategy for identifying mature lung mast cells. (E) Gating strategy for identifying mature blood basophils. Mature lung mast cells and mature blood basophils were sorted in 2 independent experiments. The photos of blood mast cell progenitors were representative of cells from 4 independent experiments, where either Lin− CD34hi CD117int/hi FcεRI+ or Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ cells were isolated.
Primary Lin− CD34hi CD117int/hi FcεRI+ mast cell progenitors express mast cell–related genes
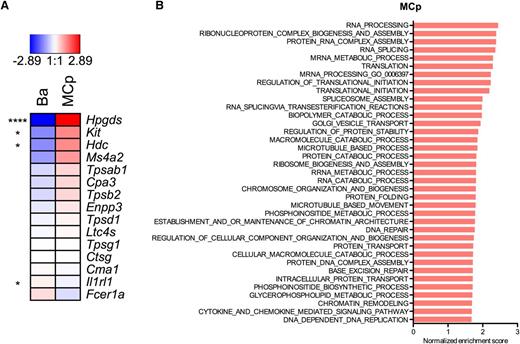
Lin− CD34hi CD117int/hi FcεRI+ mast cell progenitors and Lin− CRTH2+ FcεRI+ blood basophils cells were sorted in parallel from 3 blood donors. A whole-transcriptome microarray analysis was performed on these 2 cell populations to investigate the differential gene expression profile. Genes that were expressed highly specifically in human mast cells in the FANTOM (Functional Annotation of the Mammalian Genome) study34 were selected for evaluation. Production of prostaglandin D2 is catalyzed by hematopoietic prostaglandin D synthase. Transcripts of hematopoietic prostaglandin D synthase (Hpgds) were exceptionally abundant in mast cell progenitors, and Hpgds was the most differentially expressed gene of the whole genome (Figure 4A). Transcripts for the gene coding for CD117 (Kit) and histidine decarboxylase (Hdc), which codes for the enzyme that catalyzes the production of histamine, were higher in mast cell progenitors than in basophils. There was a strong trend toward mast cell progenitors having more transcripts of the β chain of FcεRI (Ms4a2), although not significantly. There was also a tendency that the transcripts for the mast cell α- and β-tryptases (Tpsab1 and Tpsb2) and carboxypeptidase A (Cpa3) were all expressed to a higher degree in the mast cell progenitors than in the basophils, even though there were no significant differences in these transcripts (Figure 4A). To provide further evidence for the commitment to the mast cell lineage, qRT-PCR analysis of proteases that are expressed in mast cells derived from human blood20 and serglycin (Srgn), the core protein for heparin/chondroitin sulfate E proteoglycans, was performed on the primary mast cell progenitors. Tryptase (Tpsab1/Tpsb2), carboxypeptidase (Cpa3), and serglycin (Srgn) were detected (Table 2). In conclusion, the mast cell progenitors express the mast cell–related genes Hpgds, Kit, Hdc, Tpsab1/Tpsb2, Cpa3, and Srgn.
Primary blood mast cell progenitors express mast cell–associated genes. Lin− CRTH2+ FcεRI+ mature blood basophils (Ba) and Lin− CD34hi CD117int/hi FcεRI+ blood mast cell progenitors (MCp) were analyzed using whole-transcriptome microarray analyses. The raw data were normalized using the robust multiarray method, and an empirical Bayes moderated t test was used to test for differentially expressed genes employing the computing language R (http://www.r-project.org) using the limma package available from the Bioconductor project (www.bioconductor.org). All analyses were performed according to the limma: Linear Models for Microarray Data User’s Guide (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia). (A) Differences are shown on a log2 scale. The mean values of each transcript from 3 blood donors are shown. *P < .05; ****P < .0001. (B) The results of a GSEA of the “biological process” Gene Ontology subcollection are shown for MCp. Only gene sets with a false discovery rate q value <.05 and an enrichment score >0.5 are shown.
Primary blood mast cell progenitors express mast cell–associated genes. Lin− CRTH2+ FcεRI+ mature blood basophils (Ba) and Lin− CD34hi CD117int/hi FcεRI+ blood mast cell progenitors (MCp) were analyzed using whole-transcriptome microarray analyses. The raw data were normalized using the robust multiarray method, and an empirical Bayes moderated t test was used to test for differentially expressed genes employing the computing language R (http://www.r-project.org) using the limma package available from the Bioconductor project (www.bioconductor.org). All analyses were performed according to the limma: Linear Models for Microarray Data User’s Guide (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia). (A) Differences are shown on a log2 scale. The mean values of each transcript from 3 blood donors are shown. *P < .05; ****P < .0001. (B) The results of a GSEA of the “biological process” Gene Ontology subcollection are shown for MCp. Only gene sets with a false discovery rate q value <.05 and an enrichment score >0.5 are shown.
qRT-PCR of mast cell progenitors
| Target gene . | HMC1 . | MCp1 . | MCp2 . | MCp3 . |
|---|---|---|---|---|
| Cma1 | 29 (4/4) | ud (0/3) | ud (0/4) | ud (0/3) |
| Cpa3 | 20 (4/4) | ud (0/3) | 33 (3/4) | ud (0/3) |
| Tpsab1/Tpsb2 | 14 (4/4) | 32 (2/3) | 33 (3/4) | 32 (1/3) |
| Gzmb | 22 (4/4) | ud (0/3) | 37 (1/4) | ud (0/3) |
| Srgn | 16 (4/4) | 32 (2/3) | 32 (4/4) | 33 (3/3) |
| Actb | 11 (3/3) | 32 (2/3) | 31 (3/3) | 32 (2/3) |
| Gapdh | 14 (4/4) | 35 (1/3) | 34 (3/4) | ud (0/3) |
| Target gene . | HMC1 . | MCp1 . | MCp2 . | MCp3 . |
|---|---|---|---|---|
| Cma1 | 29 (4/4) | ud (0/3) | ud (0/4) | ud (0/3) |
| Cpa3 | 20 (4/4) | ud (0/3) | 33 (3/4) | ud (0/3) |
| Tpsab1/Tpsb2 | 14 (4/4) | 32 (2/3) | 33 (3/4) | 32 (1/3) |
| Gzmb | 22 (4/4) | ud (0/3) | 37 (1/4) | ud (0/3) |
| Srgn | 16 (4/4) | 32 (2/3) | 32 (4/4) | 33 (3/3) |
| Actb | 11 (3/3) | 32 (2/3) | 31 (3/3) | 32 (2/3) |
| Gapdh | 14 (4/4) | 35 (1/3) | 34 (3/4) | ud (0/3) |
mRNA levels presented as raw CT values . Lin− CD34hi CD117int/hi FcεRI+ mast cell progenitors from 3 donors (MCp1-3) and HMC1 cells were subjected to qRT-PCR for mast cell proteases (Cma1, Cpa3, Tpsab1/Tpsb2, Gzmb), serglycin (Srgn), and the housekeeping genes β-actin (Actb) and glyceraldehyde 3-phosphate dehydrogenase (Gapdh). The mean CT values for 3 to 4 qRT-PCR analyses of the same samples are presented. The number of qRT-PCR analyses that contributed to the total number of analyses of each gene is presented in parentheses after the mean CT number. The controls without cDNA did not generate detectable signals for any of the target genes, except for Actb where the mean background CT value was 36.
ud, under detection limit in all runs.
A GSEA was performed to explore what biological processes were active in mast cell progenitors. Many of the processes were involved in protein synthesis and localization (Figure 4B).
Individuals with reduced lung function have an increased frequency of circulating mast cell progenitors
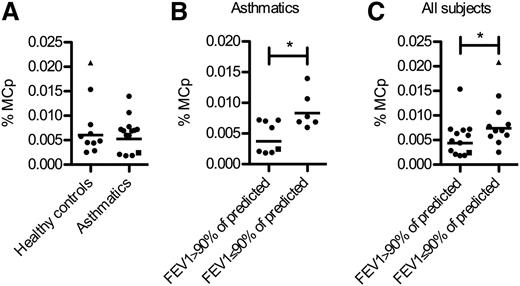
As asthmatics have an increased number of lung mast cells, we examined if this phenomenon was reflected by an increased frequency of Lin− CD34hi CD117int/hi FcεRI+ blood cells. No difference in the frequency of Lin− CD34hi CD117int/hi FcεRI+ blood mast cell progenitors was found when comparing healthy nonatopic individuals with patients with well-controlled asthma recruited in the MIDAS study23-25 (Figure 5A). Interestingly, the asthmatics with reduced lung function (FEV1 ≤90% of predicted) had a higher frequency of circulating mast cell progenitors than those with normal lung function (FEV1 >90% of predicted) (Figure 5B). The same pattern was observed among all individuals including healthy controls (Figure 5C).
The frequency of mast cell progenitors is increased in subjects with reduced lung function. The frequencies of Lin− CD34hi CD117int/hi FcεRI+ mast cell progenitors (MCp) were quantified from 24 blood donors by flow cytometry. (A) The subjects were grouped according to the classification in the MIDAS baseline study. (B) The asthmatic subjects, classified at the baseline study, were divided into 2 groups based on the prebronchodilator FEV1 values expressed as percent of the predicted at the time of blood mast cell progenitor analysis. (C) All subjects including healthy controls were divided into 2 groups based on the prebronchodilator FEV1 values expressed as percent of the predicted at the time of blood mast cell progenitor analysis. One of the healthy controls had developed asthma prior to our follow-up study when blood was sampled (shown as a triangle). One patient diagnosed with nonallergic asthma in the baseline study is shown as a square. Differences between groups were evaluated by 2-tailed unpaired Student t tests of the log-transformed data. The geometric mean values and all subjects are shown.
The frequency of mast cell progenitors is increased in subjects with reduced lung function. The frequencies of Lin− CD34hi CD117int/hi FcεRI+ mast cell progenitors (MCp) were quantified from 24 blood donors by flow cytometry. (A) The subjects were grouped according to the classification in the MIDAS baseline study. (B) The asthmatic subjects, classified at the baseline study, were divided into 2 groups based on the prebronchodilator FEV1 values expressed as percent of the predicted at the time of blood mast cell progenitor analysis. (C) All subjects including healthy controls were divided into 2 groups based on the prebronchodilator FEV1 values expressed as percent of the predicted at the time of blood mast cell progenitor analysis. One of the healthy controls had developed asthma prior to our follow-up study when blood was sampled (shown as a triangle). One patient diagnosed with nonallergic asthma in the baseline study is shown as a square. Differences between groups were evaluated by 2-tailed unpaired Student t tests of the log-transformed data. The geometric mean values and all subjects are shown.
Discussion
This study describes a rare population of Lin− CD34hi CD117int/hi FcεRI+ mast cell progenitors in human blood. As shown earlier, blood progenitors within the CD34+ CD117+ CD13+ population can give rise to mast cells.21 In agreement with these results, we found mast cell–forming potential among the Lin− CD34hi CD117int/hi CD13+ cells, especially in the FcεRI+ fraction. However, as we observed that the CD13 gate divided the Lin− CD34hi CD117int/hi FcεRI+ cell population, the CD13 marker was not further used to discriminate mast cell progenitors. In the study by Kirshenbaum et al, the CD34+ CD117+ CD13+ cells gave rise to mast cells, but also to CD117− FcεRI− CD14+ monocytes, when cultured in vitro.21 In our study, the Lin− (CD14− CD4− CD8− CD19−) CD34hi CD117int/hi FcεRI+ progenitors gave rise to few (if any) CD117− FcεRI− cells. In contrast, Lin− CD34hi CD117int/hi FcεRI− cells formed a clear population of CD117− FcεRI− cells upon culture. Therefore, it is likely that the gating of FcεRI+ cells from the Lin− CD34hi CD117int/hi progenitors separates the mast cell lineage from the monocyte lineage.
Tryptase+ (mature) mast cells without FcεRI expression have been reported in vitro,35 and mast cells without FcεRI expression can be found in vivo.36 In our investigation, the focus was to identify progenitors that give rise to CD117+ FcεRI+ mast cells. As Lin− CD34hi CD117int/hi FcεRI+ did not form a 100% pure CD117+ FcεRI+ progeny after culture, attempts to further enrich the mast cell purity were made. The Lin− CD34hi CD117int/hi FcεRI+ cells were gated based on expressions of CD45RA, CRTH2, and integrin β7. However, the progeny of the Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ cells still showed a variable phenotype with 16% to 90% CD117+ FcεRI+ cells, although nearly all were CD117+ after 7 days in vitro. Yet, apart from some dead cells, they all had an immature mast cell–like morphology. Because the sorted cells were cultured in a cytokine cocktail, it is highly unlikely that all CD117+ FcεRI− cells at day 7, formed from either Lin− CD34hi CD117int/hi FcεRI+ CD45RA− CRTH2− integrin β7+ cells or Lin− CD34hi CD117int/hi FcεRI+ cells, were multipotent progenitors. Rather, the CD117+ FcεRI− cells may constitute immature mast cells that lost FcεRI expression during the culture.
The whole-transcriptome microarray analyses verified that the Lin− CD34hi CD117int/hi FcεRI+ progenitors were expressing mast cell–related genes and showed that they were actively differentiating. The genes Hpgds, Kit, and Hdc, which are highly expressed by mast cells according to the FANTOM consortium study,34 were significantly more expressed in Lin− CD34hi CD117int/hi FcεRI+ cells than in basophils. Furthermore, mRNA for Tpsab1/Tpsb2, Cpa3, and Srgn, but not Cma1, could be detected in the mast cell progenitors using qRT-PCR. Human mast cells are divided into 2 subtypes (ie, tryptase- and chymase-positive mast cells and tryptase only–positive mast cells). Both subtypes also contain transcripts of carboxypeptidase A (Cpa3).37 Because the mast cell progenitors had undetectable levels of chymase transcripts, this expression is likely turned on later during mast cell development, although the possibility that tryptase- and chymase-positive mast cells arise from another population cannot be excluded. Altogether, the whole-genome expression microarray analyses and the qRT-PCR data support the conclusion that Lin− CD34hi CD117int/hi FcεRI+ blood cells are predominantly mast cell progenitors.
There are several similarities between mast cell progenitors in mice and humans such as expression of CD117 and integrin β7, and the majority of murine BALB/c blood mast cell progenitors express FcεRI.13 Approximately 0.0045% of the blood mononuclear cells in these mice are committed mast cell progenitors,13 which is comparable to the median value of 0.0053% Lin− CD34hi CD117int/hi FcεRI+ cells observed in healthy humans. A major difference between mice and humans is the proliferative capacity of the mast cell progenitors. Mouse mast cell progenitors undergo extensive proliferation when cultured in vitro. In fact, 1 single mast cell progenitor gives rise to roughly 103 mast cells after 7 days in culture.38 Here, human blood mast cell progenitors divided poorly. Sorted single cells became in median only 3 cells after 1 week of culture. We speculate that this may be because either an essential unknown growth factor is to be found for optimal growth in vitro or the human blood mast cell progenitors have lost a great part of their proliferative ability during their differentiation.
The ontogeny of mast cells is still to be traced. In humans, the lymphoid-primed multipotent progenitor branch is characterized by expression of CD45RA.39 Given that Lin− CD34hi CD117int/hi FcεRI+ mast cell progenitors were CD45RA−, and bipotent mast cell/monocyte progenitors have been described,21 it is reasonable to assume that the mast cell/monocyte axis branches off directly from multipotent progenitors. Studies delineating the origin of human mast cell progenitors are awaited.
As asthmatics have an increased number of mast cells in the airways,8,9,40 we determined whether this was reflected by a higher frequency of blood mast cell progenitors in asthmatics than in healthy controls. We conclude that there was no major difference in the frequency of human blood mast cell progenitors between the 10 healthy subjects and 14 asthmatics. This may reflect that the asthmatics had good asthma control. Nevertheless, when the individuals with normal lung function and those with reduced lung function were grouped among asthmatics or all individuals, those with reduced lung function had a higher frequency of circulating mast cell progenitors than those with a normal lung function. Notably, the differences were significant even for a relatively small number of individuals analyzed. High alveolar mast cell numbers are found in uncontrolled asthmatics with reduced lung function.9 Therefore, a high blood mast cell progenitor frequency may reflect ongoing recruitment to the lung resulting in increased mast cell density, which could contribute to the reduced lung function. Quantification of Lin− CD34hi CD117int/hi FcεRI+ mast cell progenitors may also be clinically important in patients with, for example, mastocytosis and parasitic infections. CD34 is lost during mast cell maturation.41 Therefore, the mast cell progenitors identified in the present study are likely more immature than CD34− CD117+ blood cells with mast cell–forming capacity quantified in mastocytosis patients.42
To summarize, we have identified a rare population of human mast cell progenitors in blood, which gives rise to predominantly mast cells, although the limited cell division capacity and partial loss of FcεRI after culture in vitro prevent us from concluding their full commitment to the mast cell lineage. However, none of our data suggest that another lineage develops from this cell population. Our finding provides a novel opportunity to phenotype immature mast cells in diseases by a blood test. It also enables the mast cell progenitors to serve as a therapeutic target in diseases characterized by an increased mast cell density in peripheral organs and as a diagnostic tool to identify aberrancies in the mast cell lineage development.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE69030).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Pia Kalm-Stephens for providing the blood samples and Birgitta Heyman for critical reading of the manuscript. The gene expression microarray analyses were performed by the Array and Analysis Facility at the Science for Life Laboratory (Uppsala Biomedical Center, Uppsala, Sweden). We performed the fluorescent-activated cell sorting on equipment provided by the BioVis Facility at the Science for Life Laboratory, Uppsala, Sweden.
This work was supported by grants from Agnes and Mac Rudberg Foundation, Lennander’s Foundation, Ellen, Walter, and Lennart Hesselman Foundation, and the Royal Swedish Academy of Sciences (J.S.D.); and from the Swedish Research Council, Malin and Lennart Philipson Foundation, the Swedish Heart-Lung Foundation, Konsul Th C Bergh Foundation, Bror Hjerpstedt Foundation, and Mats Kleberg Foundation (J.H.). The build-up of the MIDAS cohort was supported by the Swedish Governmental Agency for Innovation Systems.
Authorship
Contribution: J.S.D. and J.H. designed the experiments and wrote the manuscript; J.S.D. and H.Ö. performed the experiments; J.S.D., H.Ö., and J.H. analyzed the experiments; A.M., C.J., and K.A. were responsible for recruitment of patients and control subjects; and M.S. coordinated the collection of the lung samples.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.S.D. is Clinical Immunology and Allergy Unit, Department of Medicine, Karolinska Institutet and Karolinska University Hospital, Stockholm, Sweden.
Correspondence: Jenny Hallgren, Department of Medical Biochemistry and Microbiology, Uppsala University, Biomedical Center, Husargatan 3, 75123 Uppsala, Sweden; e-mail: jenny.hallgren@imbim.uu.se.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal