Key Points
The CHAMPION-1 study is the first clinical trial to investigate carfilzomib on a once-weekly dosing schedule with dexamethasone.
Once-weekly carfilzomib (30-minute infusion; 20 and 70 mg/m2) with dexamethasone is feasible and effective in relapsed/refractory MM.
Abstract
Carfilzomib, a proteasome inhibitor, is approved in the United States as a single agent, and in combination with dexamethasone or lenalidomide/dexamethasone (KRd) for relapsed or refractory multiple myeloma (MM). Under the single-agent and KRd approvals, carfilzomib is administered as a 10-minute IV infusion on days 1, 2, 8, 9, 15, and 16 of 28-day cycles (20 mg/m2 [cycle 1, days 1-2]; 27 mg/m2 thereafter). This multicenter, single-arm, phase 1/2 study, Community Harmonized Assessment of Myeloma Patients via an Integrated Oncology Network-1 (CHAMPION-1), evaluated once-weekly carfilzomib with dexamethasone in relapsed, or relapsed and refractory MM (1-3 prior therapies). Patients received carfilzomib (30-minute IV infusion) on days 1, 8, and 15 of 28-day cycles. The phase 1 portion used a 3 + 3 dose-escalation scheme to determine the maximum tolerated dose (MTD) of carfilzomib. During phase 2, patients received carfilzomib on the same schedule at the MTD. Patients received dexamethasone (40 mg) on days 1, 8, 15, and 22; dexamethasone was omitted on day 22 for cycles 9+. A total of 116 patients were enrolled. The MTD was 70 mg/m2, and 104 patients (phase 1/2) received carfilzomib 70 mg/m2. At 70 mg/m2, the median number of prior regimens was 1; and 52% were bortezomib-refractory. At 70 mg/m2, the most common grade ≥3 adverse events were fatigue (11%) and hypertension (7%). Overall response rate at 70 mg/m2 was 77%. Median progression-free survival was 12.6 months. These findings merit additional evaluation of the once-weekly dosing regimen. This trial was registered at www.clinicaltrials.gov as #NCT01677858.
Introduction
Patients with relapsed or refractory multiple myeloma (RRMM) have limited treatment options. For patients exposed to the proteasome inhibitor bortezomib (BTZ) and/or immunomodulatory agents (IMiDs) in the front-line setting, a clinical need remains to identify additional treatments that can improve responses in the relapsed setting. For patients with at least a partial response (PR) to prior BTZ-based therapy, retreatment with BTZ has been shown to be an acceptable treatment option, with an overall response rate (ORR) of 40% observed in a phase 2 study.1 However, patients who become refractory to BTZ and an IMiD have poor response and survival outcomes, increasing the need for effective treatment of RRMM.2
Carfilzomib is an irreversible, epoxyketone proteasome inhibitor that received accelerated approval in the United States in 2012 for the treatment of patients with relapsed and refractory multiple myeloma (MM); carfilzomib has since received approval in the United States when used in combination with dexamethasone or lenalidomide (LEN) plus dexamethasone for patients with RRMM (1 to 3 prior lines of therapy), or when used as a single agent for patients with RRMM (1 or more prior lines of therapy).3 Under the initial approvals for single-agent carfilzomib and carfilzomib with LEN and dexamethasone (KRd), carfilzomib is administered on a twice-weekly dosing schedule, infused over 10 minutes, with a starting dose of 20 mg/m2 on cycle 1 days 1 and 2, and stepped-up to a target dose of 27 mg/m2 thereafter. The accelerated approval of carfilzomib was based on a phase 2 study (PX-171-003-A1; #NCT00511238), where the ORR for response-evaluable patients (n = 257), who had failed previous BTZ and an IMiD (median of 5 prior lines of therapy), was 23.7%; the clinical benefit rate (CBR) was 37.0%.4 The approval for KRd was based on the phase 3 ASPIRE (carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone for the treatment of patients with relapsed multiple myeloma) study that demonstrated treatment with KRd significantly improved progression-free survival (PFS) vs Rd alone in relapsed MM (median, 26.3 vs 17.6 months).5
Although efficacy and safety data from studies evaluating the 20 and 27 mg/m2 dose of carfilzomib administered as a 10- or 2- to 10-minute infusions for patients with relapsed or relapsed and refractory MM supported the approvals of carfilzomib, preclinical safety results suggested that a longer, 30-minute infusion may be better tolerated and permit administration of higher doses than the 2- to 10-minute infusions.6 A subsequent phase 1 study (PX-171-007; #NCT00531284) evaluated carfilzomib administered twice weekly as a 30-minute infusion with or without dexamethasone for patients with RRMM. Carfilzomib was administered on days 1, 2, 8, 9, 15, and 16 of 28-day cycles at a starting dose of 20 mg/m2 on days 1 and 2 of cycle 1; subsequent doses were escalated in a 3 + 3 dose-escalation scheme, at 36, 45, 56, or 70 mg/m2.7 The maximum tolerated dose (MTD) of carfilzomib administered twice weekly as a 30-minute infusion was 56 mg/m2. Among patients who received single-agent carfilzomib (56 mg/m2; n = 24), the ORR was 50%. The ORR observed with carfilzomib (45 or 56 mg/m2) and dexamethasone (n = 22) was 55%. The tolerability profile of carfilzomib 56 mg/m2 was consistent with that of previous studies of carfilzomib 27 mg/m2. These findings were confirmed with the results of the randomized, phase 3 ENDEAVOR trial (RandomizEd, OpeN Label, Phase 3 Study of Carfilzomib Plus DExamethAsone Vs Bortezomib Plus DexamethasOne in Patients With Relapsed Multiple Myeloma; #NCT01568866) comparing carfilzomib plus dexamethasone vs BTZ plus dexamethasone in RRMM (N = 929), which supported the approval of carfilzomib with dexamethasone for patients with RRMM. Carfilzomib (starting dose of 20 mg/m2 on C1D1 and C1D2; 56 mg/m2 thereafter) administered twice weekly significantly increased median PFS vs BTZ (18.7 vs 9.4 months, respectively), and achieved greater ORRs (77% vs 63%) and rates of complete response (CR) or better (12.5% vs 6.2%).8
Twice-weekly IV administration of anti-myeloma therapy can be burdensome for patients, especially for those who are elderly, suffer from myeloma-related symptoms, or who live far from the clinic.
Based on findings from studies showing that BTZ administered once weekly continuously to patients with RRMM has comparable efficacy to the conventional twice-weekly administration for 2 out of the 3-week dosing regimen for this proteasome inhibitor,9,10 we wanted to determine whether a more convenient once-weekly carfilzomib regimen would show at least similar efficacy to the currently approved twice-weekly dosing schedule. We therefore conducted a phase 1/2 study to determine the MTD, and evaluate the safety and efficacy of once-weekly carfilzomib administered on days 1, 8, and 15 of 28-day cycles combined with once-weekly dexamethasone 40 mg for patients with relapsed, or relapsed and refractory MM.
Methods
Study design and treatment
Community Harmonized Assessment of Myeloma Patients via an Integrated Oncology Network-1 (CHAMPION-1) was a phase 1/2, multicenter (a collaboration of community oncology networks), single-arm, dose-escalation study investigating carfilzomib administered on a once-weekly schedule in combination with dexamethasone to patients with relapsed, or relapsed and refractory MM. The primary objective of the phase 1 portion was to determine the MTD of once-weekly carfilzomib with dexamethasone. The primary objective of the phase 2 portion was to determine the ORR (≥PR). Secondary objectives included the assessment of safety and tolerability, determination of carfilzomib pharmacokinetics (PK) parameters, and evaluations of the CBR (≥ minimal response) and PFS in the phase 2 portion.
Patients received carfilzomib as a 30-minute, IV infusion on days 1, 8, and 15 of 28-day cycles. In the phase 1, dose-escalation portion, patients received carfilzomib at 20 mg/m2 on cycle 1, day 1 (C1D1); subsequent doses (beginning with C1D8) were escalated in a standard 3 + 3 dose-escalation scheme at 45, 56, 70, or 88 mg/m2 to determine the MTD.
In the phase 2 portion, patients received carfilzomib at the MTD on the same schedule used as in the phase 1 portion (ie, 20 mg/m2 on C1D1; escalation to the phase 1 MTD thereafter). All patients received dexamethasone 40 mg IV or orally on days 1, 8, 15, and 22 of 28-day cycles for the first 8 cycles. Beginning with cycle 9, dexamethasone was omitted on day 22. Treatment was continued until progressive disease or unacceptable toxicity.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. Institutional Review Board approval was obtained at each study site. The sponsor designed the study in collaboration with the investigators, and collected, analyzed, and interpreted the data in conjunction with the investigators.
Patients
Adult patients with relapsed, or relapsed and refractory MM, who had received 1 to 3 prior regimens of therapy were eligible to enroll; additional eligibility criteria included Eastern Cooperative Oncology Group performance status 0 to 1, adequate hepatic function, left ventricular ejection fraction of ≥40%, absolute neutrophil count of ≥1000/mm3, platelet count of ≥50 000/mm3, and creatinine clearance of ≥30 mL/min. Exclusion criteria included cytotoxic chemotherapy within 28 days prior to enrollment, congestive heart failure (CHF) (in terms of eligibility criteria, defined as active CHF by New York Heart Association criteria [classes III-IV], symptomatic ischemia, or conduction abnormalities uncontrolled by conventional intervention, or myocardial infarction within 6 months prior to enrollment), grade 3 to 4 neuropathy within 14 days prior to enrollment (or grade 2 neuropathy with pain), and prior carfilzomib therapy. All patients provided written informed consent.
Assessments
Echocardiograms were conducted before study entry. Patients with risk factors for or evidence of existing heart disease were closely monitored throughout their treatment with carfilzomib. Follow-up echocardiograms were conducted for patients who developed clinically significant CHF or clinically important signs/symptoms suggesting decreased ventricular function. Patients who experienced dyspnea were evaluated for the presence of associated conditions and management was tailored to the appropriate treatment of the underlying disorder.
The incidence and severity of adverse events (AEs), coded using the Medical Dictionary for Regulatory Activities, were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Dose-limiting toxicities (DLTs) were defined as grade 4 neutropenia lasting for >7 days; febrile neutropenia of any duration; grade 4 thrombocytopenia lasting for >14 days despite holding treatment; grade 3 to 4 thrombocytopenia associated with grade >1 bleeding; grade ≥3 nonhematologic toxicity (excluding nausea, vomiting, diarrhea, fatigue lasting <14 days, increased serum creatinine, or electrolyte abnormalities that were not clinically significant and required no treatment); grade ≥3 acute kidney injury lasting >72 hours; and grade ≥3 nausea, vomiting, or diarrhea uncontrolled by maximal antiemetic/antidiarrheal medication.
The MTD of carfilzomib was defined as the highest carfilzomib dose at which <33% of patients experienced a treatment-related DLT during the first 28-day cycle of treatment in the phase 1 portion of the study. A total of 15 patients were to be enrolled at the MTD before initiating the phase 2 portion of the study.
Response to therapy was assessed according to the International Myeloma Working Group Uniform Response Criteria,11 with the addition of minimal response according to the European Group for Blood and Marrow Transplant.12
PK analyses were conducted in patients enrolled in the phase 1 and 2 portions. Blood samples for PK were collected on C1D1 and C1D15, for the 70 and 88 mg/m2 cohorts (phase 1) or C1D1 and C2D15 (phase 2), at the following time points: pre-dose, 5 and 15 minutes after the start of infusion, the end of infusion, and 5, 15, 30, 60, 120, and 240 minutes post-infusion. A validated liquid chromatography/mass spectrometry assay was used to analyze plasma samples.13 PK assessments included estimation of the area under the curve (AUC) (ie, plasma concentration time curve), maximum concentration (Cmax), clearance, and terminal half-life. PK analysis for the 20 mg/m2 dosing was derived from C1D1 blood samples.
Pharmacodynamic (PDn) analyses were conducted only in the phase 2 portion, and included the measurement of proteasome activity in whole blood and isolated peripheral blood mononuclear cells (PBMCs) via a fluorogenic substrate assay14,15 and an enzyme-linked immunosorbent assay (ProCISE).16 Blood samples were collected on C1D1 (20 mg/m2 dosing), C1D8 (70 mg/m2), and C2D1 (70 mg/m2) at pre-dose and 1 hour post-infusion time points. The fluorogenic Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (LLVY-AMC) assay was used to quantify proteolytic activity specific only to the chymotrypsin-like (CT-L) subunits of the proteasome (the primary inhibition targets of carfilzomib), whereas the ProCISE assay was employed to further elucidate inhibition of the secondary catalytic targets (caspase-like and trypsin-like proteasomal subunits), in addition to CT-L activity.
Statistical analyses
The estimated sample size for the phase 2 portion was determined using the prior power method,17 which used phase 1 data at the MTD as historical data in determining the prior distribution of ORR. A total of ≥55 responders from 90 patients in the phase 2 portion were required to provide a lower bound of a 1-sided 95% confidence interval (CI) >55%.
The safety population consisted of all patients who received at least 1 dose of study treatment; all safety and efficacy analyses were performed using this population.
Results
Patients and enrollment
From September 2012 to September 2014, a total of 116 patients were enrolled from 32 sites in the United States. Data were analyzed through June 24, 2015. Twenty-seven patients were enrolled in the phase 1, dose-escalation portion of the study, and 89 patients were enrolled in the phase 2 portion. Baseline demographics and disease characteristics for patients enrolled in both study phases, including at the MTD (see “Safety”), are presented in Table 1. Median age of all patients was 68.5 years (range, 41-88); 31 patients (27%) were ≥75 years.
Overall, patients received a median of 1 prior regimen (range, 1-3); 60 patients (52%) had 1 prior regimen. A total of 96 patients (83%) received prior BTZ, and 62 patients (53%) were BTZ-refractory (refractory was defined as disease not achieving a minimal response or better, progressing during therapy, or within 60 days after completion of therapy); 58 patients (50%) received prior LEN and 37 patients (32%) were LEN-refractory. Twenty-three percent of patients received a prior stem cell transplant; and 19% received prior radiation therapy. At baseline, 54% of patients had ISS stage II to III disease. Of patients with known cytogenetic risk status (n = 69, all in the phase 2 portion), 18 patients (26%) were classified as high risk by FISH criteria (t[4;14], t[14;16], or deletion 17p). Median carfilzomib treatment duration for all patients was 7.8 months (range, 0.03-31.6).
DLTs
During phase 1, in the dose-escalation portion of the study, no DLTs were observed in the 45, 56, or 70 mg/m2 dose cohorts. In the 88 mg/m2 dose cohort (n = 6), 2 patients experienced a DLT during cycle 1: grade 3 dyspnea and grade 3 vomiting (n = 1 each). Per study protocol, the next lowest dose cohort (70 mg/m2) initially was expanded to include an additional 3 patients and subsequently expanded to include a further 9 patients. In this expansion cohort, 1 patient experienced a DLT of grade 3 dyspnea. The MTD of once-weekly carfilzomib with dexamethasone in the CHAMPION-1 study was determined to be 70 mg/m2.
Safety
Among patients treated at the MTD (phase 1 and phase 2; n = 104), the median carfilzomib treatment duration was 7.7 months (range, 0.03-24.15). All patients treated at the MTD experienced at least 1 treatment-emergent AE, and 64 patients (62%) experienced at least 1 grade ≥3 AE. The most common nonhematologic AEs of any grade at the MTD were fatigue (53%), nausea (37%), insomnia (32%), headache (31%), and diarrhea (31%) (Table 2). Commonly occurring hematologic AEs of any grade included anemia (28%), thrombocytopenia (22%), and neutropenia (10%). Common grade ≥3 nonhematologic AEs were fatigue (11%), hypertension (7%), pneumonia (6%), and acute kidney injury (6%) (Table 3). The most common grade ≥3 hematologic AEs were thrombocytopenia (6%), anemia (6%), and neutropenia (4%). Rates of grade ≥3 dyspnea, cardiac failure, and peripheral neuropathy were 5%, 2%, and 1%, respectively.
Sixteen patients (15%) treated at the MTD had at least 1 carfilzomib dose reduction due to AEs; among all patients treated at the MTD, median carfilzomib dose per administration was 66.8 mg/m2 (ie, 95.4% of the planned 70 mg/m2 dose). The AEs that led to carfilzomib dose reductions included dyspnea, pneumonitis, fatigue, malaise, headache, paresthesia, peripheral sensory neuropathy, syncope, angina pectoris, CHF, palpitations, tachycardia, arthralgia, muscle spasms, flatulence, blurred vision, elevated blood creatinine levels, insomnia, and acute kidney injury (patients may have had >1 AE that resulted in a dose reduction).
At the MTD, 36 patients (35%) discontinued study treatment due to progressive disease, 14 (13%) due to patient decision (see supplemental Table 1, available on the Blood Web site; 3 patients provided the reason as being related to AEs or toxicity: 1 to pneumonia, 1 to AEs, and 1 to toxicities), 12 (12%) due to AEs, and 11 (11%) due to investigator discretion (supplemental Table 1; 1 patient provided the reason as being related to poor tolerability), and 1 (1%) due to death. The AEs leading to treatment discontinuation included acute myocardial infarction, atrial fibrillation, cardiorespiratory arrest, headache, ischemic stroke, peripheral neuropathy, asthenia, disease progression, fatigue, acute kidney injury, increased blood creatinine levels, decreased ejection fraction, and acute respiratory distress syndrome (ARDS) (n = 1 each except for acute kidney injury [n = 3]; patients may have had >1 AE resulting in treatment discontinuation).
A total of 5 patients died on study; causes of death were reported as disease progression (n = 1) and AEs (acute kidney injury, acute respiratory failure, ARDS, and cardiopulmonary arrest [n = 1 each]; the last 2 AEs were considered carfilzomib-related). All 5 deaths occurred at the 70 mg/m2 dose level in the phase 2 portion of the study.
Efficacy
All 104 patients treated at the MTD (phase 1 and 2) were analyzed for response. The ORR was 77% (95% CI, 68 to 85); 14 patients (13%) achieved ≥ CR, including 3 (3%) with a stringent CR. Forty-eight patients (46%) had a very good PR or better (Table 4). The CBR was 84% (95% CI, 75 to 90). The ORRs for BTZ-exposed, BTZ-refractory, and LEN-refractory patients were 73%, 63%, and 71%, respectively. The ORR for patients with 2 or 3 prior regimens was 68%. Median time to response for patients who achieved ≥PR was 1.6 months (range, 0.7-7.2).
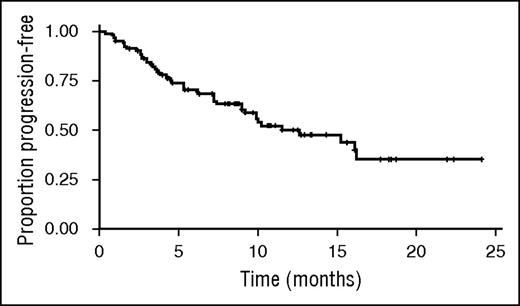
Kaplan–Meier estimated median PFS was 12.6 months (95% CI, 9.0-not estimable); median follow-up for PFS was 10.7 months (95% CI, 9.2-12.5) (Figure 1).
Kaplan–Meier estimate of PFS. Kaplan–Meier estimated median PFS was 12.6 months (95% CI, 9.0-not estimable); median follow-up for PFS was 10.7 months (95% CI, 9.2-12.5).
Kaplan–Meier estimate of PFS. Kaplan–Meier estimated median PFS was 12.6 months (95% CI, 9.0-not estimable); median follow-up for PFS was 10.7 months (95% CI, 9.2-12.5).
PKs and PDns
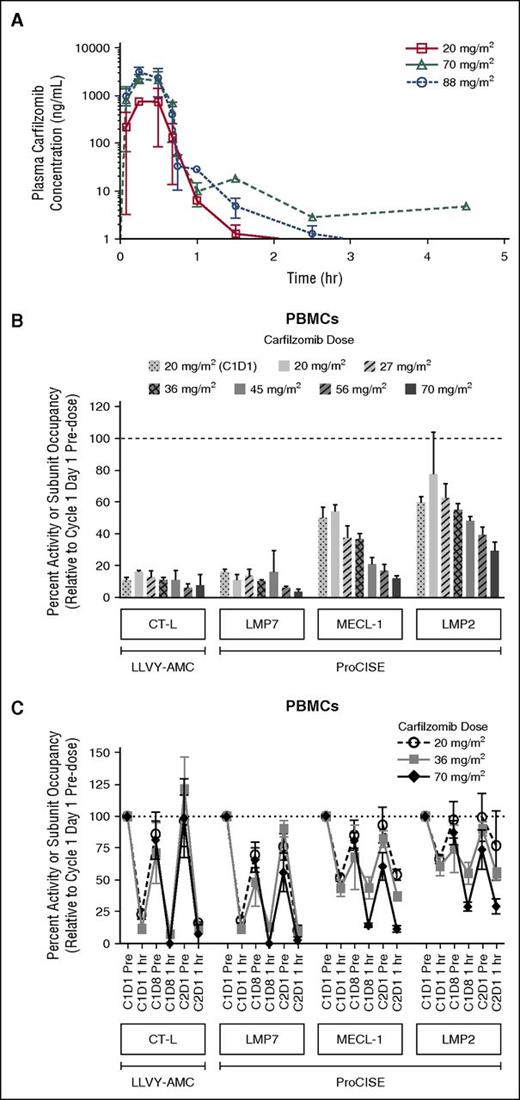
The PK results from this study indicated that when administered once weekly as a 30-minute infusion, carfilzomib had a mean terminal half-life of ≤1 hour across all dose levels. Peak carfilzomib concentrations generally occurred near the end of the 30-minute infusion (Figure 2A). There was an approximate dose-proportional increase in the geometric mean Cmax and AUC from 20 to 88 mg/m2 (Table 5). The mean AUC and Cmax following a 70 mg/m2 dose were 1050 ng × h/mL and 2510 ng/mL, respectively.
Carfilzomib plasma concentration-time profile following a 30-minute IV infusion of carfilzomib, and proteasome activity and active-site binding probe subunit occupancy in PBMCs. (A) Mean (standard deviation) carfilzomib plasma concentration time profile after administration via a 30-minute IV infusion of 20, 70, or 88 mg/m2 carfilzomib. (B) Dose-dependent proteasome activity and active-site binding probe subunit occupancy in PBMCs at 1 hour post-dose on C2D1 (unless otherwise noted) in CHAMPION-1 and PX-171-007; 20 mg/m2 (C1D1), n = 10-11; 20 mg/m2, n = 3; 27 mg/m2, n = 3; 36 mg/m2, n = 20-24; 45 mg/m2, n = 3-8; 56 mg/m2, n = 5-13; and 70 mg/m2, n = 7. Data for 20, 27, 36, 45, and 56 mg/m2 doses are from the PX-171-007 study (twice-weekly dosing)7 ; 20 mg/m2 (C1D1 only) and 70 mg/m2 data are from the CHAMPION-1 study (once-weekly dosing). Values (relative to C1D1 pre-dose) are presented as mean (± standard error of the mean). CT-L activity was measured by the fluorescent enzymatic cleavage LLVY-AMC assay,14,15 whereas subunit occupancy was measured by the ProCISE assay.16 The ProCISE assay employs a proteasome active-site binding probe that generates a signal from catalytic sites that are not already bound by inhibitor (ie, carfilzomib); subunit (probe) occupancy is thus proportional to the presence of active subunit. (C) Time-course of inhibition, and recovery of proteasome activity and active-site binding probe subunit occupancy in peripheral blood at 1 hour post-dose on C2D1; 20 mg/m2, n = 3-48; 36 mg/m2, n = 4-24; and 70 mg/m2; n = 7-11. LMP2, low molecular mass polypeptide 2; LMP7, low molecular mass polypeptide 7; MECL-1, multi-catalytic endopeptidase complex-like 1.
Carfilzomib plasma concentration-time profile following a 30-minute IV infusion of carfilzomib, and proteasome activity and active-site binding probe subunit occupancy in PBMCs. (A) Mean (standard deviation) carfilzomib plasma concentration time profile after administration via a 30-minute IV infusion of 20, 70, or 88 mg/m2 carfilzomib. (B) Dose-dependent proteasome activity and active-site binding probe subunit occupancy in PBMCs at 1 hour post-dose on C2D1 (unless otherwise noted) in CHAMPION-1 and PX-171-007; 20 mg/m2 (C1D1), n = 10-11; 20 mg/m2, n = 3; 27 mg/m2, n = 3; 36 mg/m2, n = 20-24; 45 mg/m2, n = 3-8; 56 mg/m2, n = 5-13; and 70 mg/m2, n = 7. Data for 20, 27, 36, 45, and 56 mg/m2 doses are from the PX-171-007 study (twice-weekly dosing)7 ; 20 mg/m2 (C1D1 only) and 70 mg/m2 data are from the CHAMPION-1 study (once-weekly dosing). Values (relative to C1D1 pre-dose) are presented as mean (± standard error of the mean). CT-L activity was measured by the fluorescent enzymatic cleavage LLVY-AMC assay,14,15 whereas subunit occupancy was measured by the ProCISE assay.16 The ProCISE assay employs a proteasome active-site binding probe that generates a signal from catalytic sites that are not already bound by inhibitor (ie, carfilzomib); subunit (probe) occupancy is thus proportional to the presence of active subunit. (C) Time-course of inhibition, and recovery of proteasome activity and active-site binding probe subunit occupancy in peripheral blood at 1 hour post-dose on C2D1; 20 mg/m2, n = 3-48; 36 mg/m2, n = 4-24; and 70 mg/m2; n = 7-11. LMP2, low molecular mass polypeptide 2; LMP7, low molecular mass polypeptide 7; MECL-1, multi-catalytic endopeptidase complex-like 1.
The PDn results from the ProCISE assay showed that in PBMCs (an immunoproteasome-enriched cellular population), the CT-L catalytic subunit (LMP7) was inhibited most strongly, followed by the trypsin-like (MECL-1) and caspase-like (LMP2) subunits, respectively (Figure 2B). One hour post-dosing, more profound LMP7 inhibition was observed following the first 70 mg/m2 C2D1 dose (97% inhibition) than after the 20 mg/m2 C1D1 dose (85%). CT-L inhibition, as assessed by the LLVY-AMC assay and dominated by LMP7 activity within immunoproteasome-rich cell types, showed similar levels of inhibition as LMP7 at C2D1 (93%) and at C1D1 (89%). MECL-1 inhibition in PBMCs was also enhanced between the 20 and 70 mg/m2 doses (from 50% to 89%), as was LMP2 inhibition (from 40% to 70%). The 20 and 70 mg/m2 data from the CHAMPION-1 study with once-weekly administration were compared with data from the 20, 27, 36, 45, and 56 mg/m2 doses of the PX-171-007 trial with twice-weekly administration7 to obtain a more complete picture of dose-dependent PDn (Figure 2B). In the PX-171-007 study, LMP7 inhibition 1 hour following the 20 mg/m2 dose on C2D1 was 89% (84% by LLVY-AMC), which was similar to that observed for the same dose in the CHAMPION-1 trial on C1D1. Inhibition of the secondary target subunits, MECL-1 and LMP2, was also comparable between the 2 studies at the 20 mg/m2 dose level. In combining trends from the PX-171-007 and CHAMPION-1 trials, although LMP7 inhibition was generally greater than 85% across all doses, a more pronounced dose-dependent increase in inhibition was observed for MECL-1 and LMP2 (approximately doubling over the 20 to 70 mg/m2 dose range). Time-course investigations demonstrated that once-weekly dosing of 70 mg/m2 resulted in a greater depth of secondary subunit (MECL-1 and LMP2) inhibition compared with that observed following twice-weekly dosing of 20 or 36 mg/m2, as noted on C1D8 and C2D1 (Figure 2C). Notably, 70 mg/m2 on a once-weekly schedule exhibited less recovery of proteasome inhibition to baseline by C2D1, compared with that displayed by the 20 or 36 mg/m2 dose levels.
Discussion
The CHAMPION-1 study is the first clinical trial to investigate carfilzomib on a once-weekly dosing schedule with dexamethasone. In the phase 1 portion, the MTD of once-weekly carfilzomib infused over 30 minutes with dexamethasone was determined to be 70 mg/m2. The infusion time of 30 minutes used with this higher dose is longer than the regulatory approved time of 10 minutes. Previous studies have suggested that a longer infusion time is associated with improved tolerability,7 and PDn studies have shown that higher doses combined with longer infusion times resulted in increased proteasome inhibition possibly resulting in improved efficacy.7,18
A total of 104 patients were treated at this dose level with a median relative dose intensity of >95%. The weekly accumulated dose of the once-weekly 70 mg/m2 dose regimen (starting dose of 20 mg/m2 on C1D1, 70 mg/m2 thereafter) is higher than that of the twice-weekly 27 mg/m2 dose regimen (starting dose of 20 mg/m2 on C1D1 and C1D2, 27 mg/m2 thereafter) currently approved in the United States (27 mg/m2 administered twice-weekly; cumulative 54 mg/m2 per week), but is less than the twice-weekly 56 mg/m2 dose regimen (starting dose of 20 mg/m2 on C1D1 and C1D2; 56 mg/m2 thereafter) used in ENDEAVOR. Despite the higher daily dose and higher cumulative dose per week compared with the 27 mg/m2 regimen, the 70 mg/m2 regimen had an overall favorable safety and tolerability profile, with only 12% of patients discontinuing treatment due to AEs. Encouraging efficacy was also observed, despite the lower weekly cumulative dose compared with the 56 mg/m2 twice-weekly regimen.
Although caution is warranted when making cross-trial comparisons, in this trial, rates of any grade and grade ≥3 AEs were similar to, or lower than, those reported in an integrated safety summary of previous phase 2 clinical trials of single-agent carfilzomib (15, 20, or 27 mg/m2) administered twice-weekly; however, patients who were enrolled in this trial were less heavily pretreated than those included in the integrated safety summary (median of 1 vs 4 prior regimens of therapy, respectively).19 The rates of any grade and grade ≥3 AEs were also similar to, or lower than, those reported in the carfilzomib/dexamethasone arm of the phase 3 ENDEAVOR study that investigated carfilzomib (56 mg/m2) plus dexamethasone vs BTZ plus dexamethasone.8 Grade ≥3 cardiac failure (2%) occurred at a lower frequency than reported in recent phase 3 studies of twice-weekly carfilzomib combination regimens (which used 10- or 30-minute infusions).5,8 However, patients in this study were less heavily pretreated than those in the phase 3 studies (median of 1 vs 2 prior therapies, respectively), which limits the conclusions that can be drawn from these comparisons. Two patients died on study due to carfilzomib-related AEs (ARDS and cardiopulmonary arrest); both had a history of hypertension and developed pneumonia on study.
It has been hypothesized that the more convenient, once-weekly dosing schedule would improve patient compliance and result in patients remaining on treatment longer than the twice-weekly dosing. This has been shown in studies of alternative dosing schedules with other anti-myeloma regimens.9,20-22 Improved patient compliance and longer treatment duration in turn may potentially result in better patient outcomes. A post hoc analysis from a phase 3 study determined that for patients with newly diagnosed MM receiving BTZ, melphalan, and prednisone, the use of once-weekly BTZ dosing was associated with lower rates of nonhematologic AEs (35% vs 51%) and a comparable 3-year PFS rate (50% vs 47%), 3-year overall survival rate (88% vs 89%), and CR rate (30% vs 35%) compared with patients who received twice-weekly BTZ dosing in the same study.9 However, the reduction in AEs also may reflect the lower amount of BTZ administered monthly using the once-weekly regimen compared with the conventional schedule. In regards to clinical development of the schedule for the oral proteasome inhibitor ixazomib, the results from 2 parallel phase 1 studies of its administration as a single agent for patients with relapsed/refractory MM suggested that the once-weekly dosing regimen with this orally administered proteasome inhibitor was associated with lower rates of any-grade skin/subcutaneous tissue disorders (22% vs 40%) and dose reductions due to AEs (32% vs 45%) and similar ORRs (18% vs 15%), compared with the twice-weekly ixazomib dosing regimen.23,24 These findings have led to adopting the once-weekly dosing regimen for use in subsequent phase 3 studies evaluating ixazomib for MM patients.24
Promising antitumor activity was observed with weekly carfilzomib and dexamethasone in this study. The ORR at the MTD was 77% (95% CI, 68 to 85), with 13% of patients achieving a CR or better. Furthermore, median Kaplan–Meier estimated PFS was 12.6 months (95% CI, 9.0-not estimable).
The PK and PDn results from the CHAMPION-1 study are supportive of a once-weekly, 70 mg/m2 regimen as an effective dose and schedule for carfilzomib. The current study showed that this regimen delivered higher weekly AUC exposure compared with that provided by the current approved dose regimen (27 mg/m2).4 Furthermore, once-weekly dosing at the MTD of 70 mg/m2 does not appear to compromise carfilzomib efficacy, given that the desired AUC exposure can be achieved and that carfilzomib is an irreversible proteasome inhibitor. The PDn data demonstrated that once-weekly dosing of 70 mg/m2 (first introduced at C1D8) not only produced a greater depth of proteasome subunit inhibition compared with twice-weekly dosing of 20 and 36 mg/m2 in the PX-171-007 study (particularly of the secondary targets, MECL-1 and LMP2), but also sustained inhibition with less recovery to baseline by C2D1.
Although caution is warranted when making cross-trial comparisons, response rates from the CHAMPION-1 study compare favorably with those of a phase 2 study of single-agent, weekly BTZ (ORR, 55%),10 with the weekly oral proteasome inhibitor ixazomib used as a single agent (ORR, 18% to 34%),23,25 in combination with LEN/dexamethasone (ORR of 78%)26 or in heavily pretreated patients with ixazomib plus dexamethasone (ORR of 31% to 51%),27 and with response rates of previous studies of twice-weekly, single-agent carfilzomib for patients with relapsed and/or refractory MM (24% to 50%).4,7 In the randomized, phase 3 study ENDEAVOR, carfilzomib (56 mg/m2 twice weekly for a cumulative dose of 112 mg/m2 per week) plus dexamethasone was associated with a statistically significant improvement in PFS compared with BTZ plus dexamethasone for patients with relapsed MM (median PFS, 18.7 vs 9.4 months; P < .0001).8 The shorter median PFS in the CHAMPION-1 study than in the carfilzomib/dexamethasone group in the ENDEAVOR study (12.6 vs 18.7 months, respectively) may reflect differences in the patient populations. A higher proportion of patients in the CHAMPION-1 study (70 mg/m2 cohort) were BTZ-refractory or had prior LEN exposure than in the carfilzomib/dexamethasone group in the ENDEAVOR study (BTZ-refractory, 52% vs 3% and LEN-exposed, 53% vs 38%, respectively).8 Additionally, a higher proportion of patients aged ≥75 years were enrolled in the CHAMPION-1 study (70 mg/m2 cohort) than in the carfilzomib/dexamethasone group in the ENDEAVOR study (28% vs 17%, respectively).8 Overall, the efficacy data from the CHAMPION-1 study, including an ORR of 77% are encouraging, despite the limitations of an open-label, single-arm design, and the relatively short follow-up for PFS.
In conclusion, the results of the phase 1/2 CHAMPION-1 study suggest that once-weekly administration of carfilzomib as a 30-minute IV infusion dose of 20 or 70 mg/m2 in combination with dexamethasone appears to be feasible, generally well tolerated, and active for patients with relapsed, or relapsed and refractory MM. This regimen is currently being examined in the ongoing phase 3 superiority study ARROW (Randomized, Open-label, Phase 3 Study in Subjects with Relapsed and Refractory Multiple Myeloma Receiving Carfilzomib in Combination with Dexamethasone, Comparing Once-Weekly versus Twice-weekly Carfilzomib Dosing, #NCT02412878), comparing the efficacy and safety of carfilzomib administered using the once-weekly 70 mg/m2 regimen vs the twice-weekly 27 mg/m2 regimen for patients with RRMM. Additional evaluation of the carfilzomib dosing regimen used in CHAMPION-1 in earlier lines of treatment and in combination therapy is also warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the patients, families, caregivers, research nurses, study coordinators, and support staff who contributed to this study, and Jessica Johnson for providing assistance with the PK analyses and reporting.
This study was sponsored by Onyx Pharmaceuticals, Inc., an Amgen subsidiary, South San Francisco, CA. Medical writing and editorial assistance was provided by BlueMomentum, an Ashfield Company, part of UDG Healthcare PLC, and funded by Onyx Pharmaceuticals, Inc., an Amgen subsidiary.
Authorship
Contribution: J.R.B., R.M.R., and P.P. designed the research; all authors performed the research; S.D. performed the statistical analysis; J.R.B., J.G.B., P.P., Y.O., J.A., S.A., and S.D., analyzed and interpreted the data; J.R.B. wrote the initial draft of the manuscript and edited the final draft; A.C., A.B., R.M.L., W.H., D.T., R.A., R.B., M.C., R.A.M., R.M.R., P.P., S.D., Y.O., J.A., S.A., and J.G.B. reviewed the draft manuscript; and all authors approved the final version for submission.
Conflict-of-interest disclosure: J.R.B. has served as a consultant for and has received research funding and honoraria from Onyx Pharmaceuticals, Inc.; R.M.L. has served as a consultant for and has received honoraria from Amgen, Incyte, Bristol-Myers Squibb, and Novartis; W.H. has served as a consultant for Onyx; R.B. has received honoraria from and has served as a consultant for Celgene, Amgen/Onyx, Genentech, and Eisai, and has served as a speakers bureau participant for Gilead, Celgene, Amgen/Onyx, Genentech, and Eisai; R.A.M. has received research funding and honoraria from Onyx; R.M.R. has served as a consultant for Celgene, Millennium/Takeda, and Amgen/Onyx; P.P., S.D., S.A., Y.O., and J.A. report equity ownership and employment with Amgen/Onyx; and J.G.B. has received research funding from MEI, Takeda, Celgene, Bristol-Myers Squibb, Onyx, Janssen, Novartis, AbbVie, Curis, Acetylon, and Array. The remaining authors declare no competing financial interests.
Correspondence: James R. Berenson, Institute for Myeloma and Bone Cancer Research, 9201 W. Sunset Blvd, Suite 300, West Hollywood, CA 90069; e-mail: jberenson@berensononcology.com.