In this issue of Blood, Page et al show that treatment with rituximab at the time of an initial episode of thrombotic thrombocytopenic purpura (TTP) is associated with a dramatic reduction in the risk of subsequent relapse. Their data raise the question of whether all or selected patients with a first episode of acquired TTP should be treated with adjuvant rituximab to prevent recurrence.1
Acquired TTP is a thrombotic microangiopathy attributable to autoantibody-mediated deficiency of the von Willebrand factor–cleaving protease, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13). Standard therapy with plasma exchange (PEX), supplemented with corticosteroids, is lifesaving. PEX works by removing anti-ADAMTS13 autoantibody and ultralarge von Willebrand factor multimers and by replenishing ADAMTS13. It does nothing, however, to address the root problem: production of pathogenic autoantibodies. It is thus not surprising that relapse is common in acquired TTP. In the Oklahoma TTP Registry, 34% of patients with TTP accompanied by severe ADAMTS13 deficiency (<10%) at initial presentation relapsed over a median of 4.7 years.2
Notwithstanding the considerable risk of relapse, the conventional approach to management of patients after recovery from a first episode of TTP is reactive rather than proactive. Patients are monitored during remission, and PEX and corticosteroids are reinstituted if and when relapse occurs. Adjuvant therapy to reduce the risk of relapse is not part of the current paradigm.
New data from the Oklahoma Registry call this approach into question.1 Page et al conducted a cohort study of 41 consecutive patients presenting with a first episode of acquired TTP from 2003 through 2014. Four of the patients died during their initial episode. Of the 37 surviving subjects, 21 (57%) were treated with standard therapy (PEX and corticosteroids) alone and 16 (43%) received rituximab in addition to standard therapy. The rituximab and nonrituximab groups were similar with respect to demographic characteristics and clinical features at presentation, but the rituximab group had more recalcitrant disease. In 14 of the 16 rituximab-treated patients, rituximab was added because of inadequate response to standard therapy. Patients in the rituximab group received more PEX treatments (median 16 vs 8, P < .01) and greater cumulative doses of corticosteroids (median 3975 mg in prednisone equivalents vs 2135, P < .01).
Although their disease was more intractable at initial presentation, patients in the rituximab group had a significantly lower rate of relapse than the standard therapy group during follow-up (P = .009). Two of 16 patients (13%) in the rituximab group relapsed at 2.5 and 9.9 years, whereas 9 of 21 patients (43%) in the standard therapy group relapsed at a median of 3.1 years (range 0.4-5.9 years). Other studies have also demonstrated reduced relapse rates with rituximab, although these investigations were limited by inclusion of both de novo and relapsed patients, use of historical rather than contemporaneous nonrituximab-treated controls, and variable follow-up.3-5
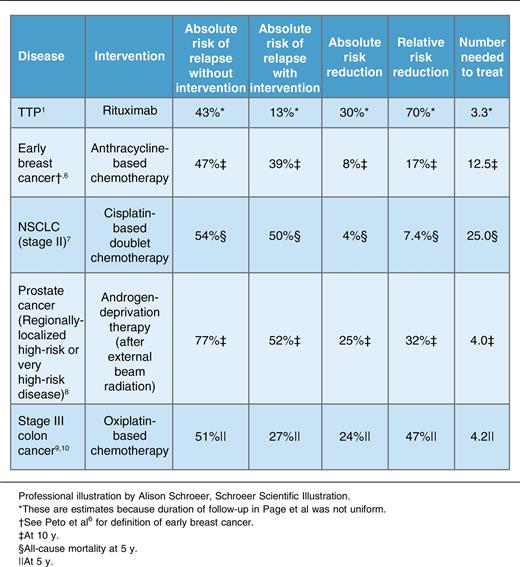
Although the duration of follow-up was not uniform among patients in the current study,1 it was similar between treatment groups, permitting estimation of risk reduction and number needed to treat. As shown in the table, rituximab was associated with an estimated 30% reduction in the absolute risk of TTP relapse and a number needed to treat of 3.3 to prevent 1 relapse. These estimates compare favorably with common oncologic settings in which adjuvant therapy is considered the standard of care (see table).6-10 For example, adjuvant treatment with a cisplatin-based doublet in patients with stage II non–small cell lung cancer reduces the absolute risk of relapse by 4%, corresponding to a number needed to treat of 25.7 Returns on adjuvant therapy are greater for early breast cancer, regionally localized high-risk prostate cancer, and stage III colon cancer,6,8-10 but still fall short of the impressive risk reduction associated with rituximab in TTP.
Of course, the analogy between adjuvant therapy for TTP and cancer is imperfect. The estimates for oncologic disease shown in the table are derived from randomized controlled trials of much larger numbers of patients. Although TTP relapses are highly disruptive to patients’ lives and carry potential long-term sequelae including depression and cognitive impairment, they are much less likely to be fatal than oncologic relapses. Rituximab is better tolerated and less toxic than most antineoplastic regimens.
In spite of these differences, mounting evidence,1,3-5 placed in the context of oncologic standards of care (see table), suggests that it may be time to move from a reactive approach to proactive treatment with adjuvant rituximab to minimize the risk of TTP relapse. More data are needed to determine whether adjuvant treatment is appropriate for all patients or only those with risk factors for relapse (eg, previous relapse, ADAMTS13 <10% in remission). Like oncologists, clinicians who treat TTP with adjuvant rituximab must accept that some patients who are destined not to relapse will needlessly be treated, but to the benefit of as many as 1 in 3 to 4 patients, in whom relapse will be averted.
Conflict-of-interest disclosure: The author declares no competing financial interests.