Key Points
Pak2 kinase activity and β-Pix interaction regulate HSPC directional migration, actin remodeling, homing, and engraftment.
Pak2 regulates homing of HSPCs to the bone marrow via CDC42 activation.
Abstract
Cytoskeletal remodeling of hematopoietic stem and progenitor cells (HSPCs) is essential for homing to the bone marrow (BM). The Ras-related C3 botulinum toxin substrate (Rac)/cell division control protein 42 homolog (CDC42) effector p21-activated kinase (Pak2) has been implicated in HSPC homing and engraftment. However, the molecular pathways mediating Pak2 functions in HSPCs are unknown. Here, we demonstrate that both Pak2 kinase activity and its interaction with the PAK-interacting exchange factor-β (β-Pix) are required to reconstitute defective Pak2Δ/Δ HSPC homing to the BM. Pak2 serine/threonine kinase activity is required for stromal-derived factor-1 (SDF1α) chemokine-induced HSPC directional migration, whereas Pak2 interaction with β-Pix is required to regulate the velocity of HSPC migration and precise F-actin assembly. Lack of SDF1α-induced filopodia and associated abnormal cell protrusions seen in Pak2Δ/Δ HSPCs were rescued by wild-type (WT) Pak2 but not by a Pak2-kinase dead mutant (KD). Expression of a β-Pix interaction-defective mutant of Pak2 rescued filopodia formation but led to abnormal F-actin bundles. Although CDC42 has previously been considered an upstream regulator of Pak2, we found a paradoxical decrease in baseline activation of CDC42 in Pak2Δ/Δ HSPCs, which was rescued by expression of Pak2-WT but not by Pak2-KD; defective homing of Pak2-deleted HSPCs was rescued by constitutive active CDC42. These data demonstrate that both Pak2 kinase activity and its interaction with β-Pix are essential for HSPC filopodia formation, cytoskeletal integrity, and homing via activation of CDC42. Taken together, we provide mechanistic insights into the role of Pak2 in HSPC migration and homing.
Introduction
The success of hematopoietic stem and progenitor cell (HSPC) engraftment in clinical transplantation may be enhanced significantly by improving homing of these cells to the bone marrow (BM) after transplantation. Retention of HSPCs in the BM and reconstitution of hematopoiesis is critically dependent on the interaction of HSPCs with 1 or several specialized microenvironments (so-called “niches”) in the BM.1 The crucial intracellular pathways triggered by these interactions are less well characterized, and how they are coordinated to regulate HSPC localization relative to niche components and subsequent survival and proliferation of HSPCs is not known. We and others have identified the Rho GTPase signaling pathway, specifically the GTPases Ras-related C3 botulinum toxin substrate (Rac) and cell division control protein 42 homolog (CDC42), as critical components of hematopoietic stem cell (HSC) engraftment and retention, integrating chemokine and adhesion signals in these cells.2-6 Rac and CDC42 regulate both interaction with the HSC niche via cytoskeleton rearrangements and modulation of proliferation and survival of these cells via kinase pathway activation. However, dissecting this complexity and defining the functional interactions between GTPases, the guanine exchange factors (GEFs) that activate them and effectors that subserve specific functions in HSCs remains a major challenge because GEFs are promiscuous in vitro and effectors are both cell and agonist specific.
Both the upstream activators of Rac activity such as the GEFs Vav1 and p210 BCR/ABL and the downstream target of Rac p21-activated kinases (Paks) are complex, multidomain proteins that regulate both Rac and other downstream effectors.7,8 We have previously demonstrated that genetic deletion of Pak2 leads to abnormalities of homing, retention, and engraftment in vivo.9 Pak proteins are serine/threonine kinases that function as downstream effectors of Rac and CDC42.10 Pak2 regulation of cytoskeletal organization is complex and involves the C-terminal kinase catalytic domain and the N terminus that mediates protein-protein interaction, including the p21-binding domain (PBD) and an autoinhibitory domain.11,12 N-terminal interacting proteins include β-Pix that appears to be involved in scaffolding a multiprotein complex made up of Rac and Pak to the membrane.13-17 Pak mediates cytoskeletal changes including stress fiber dissolution, lamellipodia formation, and focal adhesion disassembly.18 The effects on the cytoskeleton are complex and can be dependent or independent of kinase activity,19 the latter mediating the scaffold function that is dependent on proline-rich domains and interaction with β-Pix.15,17 Pak has previously been implicated in T cells and mast cell responses to stromal-derived factor-1 (SDF1) and facilitates activation of Ras via Raf1.20 Although Pak2 has been implicated in a variety of nonhematopoietic cell lines, in various cancers and in T cells and mast cells, there are no data on the role of specific Pak2 domains in HSPCs.
The knowledge of the pathways regulated by these domains that are critical for homing and engraftment and sustained hematopoiesis will likely lead to the identification of new targets for intervention and manipulation. Here, we show that HSC regulation of homing and engraftment requires both Pak2 kinase activity and its interaction with the exchange factor β-Pix. These interactions distinctly regulate response to the chemokine SDF1 and extracellular matrix protein, fibronectin, via cell shape changes, directional migration, and homing via Pak2 kinase-dependent CDC42 activation. By understanding the function of these domains in the specific cellular context of engraftment and the requirements of Rac-Pak2-CDC42 signaling that control HSPC function, a more precise definition and understanding of homing to the BM has been obtained.
Methods
Cloning
The PCR-amplified Cre-T2A-HA fragment was cloned in frame with Pak2 complementary DNA (cDNA; pDONR223-Pak2) (Addgene plasmid #23655)21 and the resulting Cre-T2A-HA-Pak2 polycistronic open reading frame was cloned into SacII and XhoI sites of murine stem cell virus (MSCV) retroviral vector8 with internal ribosome entry site (IRES)–green fluorescent protein (GFP) as a selection marker. The domain mutants of Pak2 protein were generated by site-directed mutagenesis (Quick Change Mutagenesis kit; Agilent, Santa Clara, CA) in the Pak2. All clones were confirmed by sequencing.
Mice and genotyping
Pak2fl/fl mice and the genotyping method were described previously.9 C57Bl/6J and nonobese diabetic/severe combined immunodeficient and interleukin-2γ receptor-deficient (NSG) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All procedures involving mice were performed according to Boston Children’s Hospital Institutional Animal Care and Use Committee guidelines.
Isolation, retroviral transductions of LSK cells
Bone marrow Lin−Kit+Sca1+ (LSK) cell isolation and culture/prestimulation was described previously.9 Prestimulated LSKs were placed on non-tissue-culture–coated plates that were precoated (fibronectin, CH296 fragment) and preloaded with the respective retroviruses. Thirty-six hours posttransduction, viable fluorescent cells were sorted using FACS Aria I (BD Biosciences, San Jose, CA), in phosphate-buffered saline (PBS) with 2% fetal calf serum (FCS) and Sytox Blue (Invitrogen, Carlsbad, CA).
Homing
The homing assays were performed as described previously.9
Long-term reconstitution assays
Adult NSG recipient mice were sublethally irradiated (2.8 Gy). For transplantation, 1 × 105 GFP+ sorted cells were injected into the tail vein. Sixteen weeks after transplantation, the peripheral blood was obtained via the retro-orbital vein bleed of individual recipient mice, subjected to red cell lysis, and analyzed by fluorescence-activated cell sorter (FACS) for GFP expression.
Western blotting, immunoprecipitation
Protein extracts from transduced, sorted 32Dcl3, or LSK cells were prepared as described previously.22 For immunoprecipitation of HA-Pak2, 1 mg of protein lysate was incubated with hemagglutinin (HA) beads for 3 hours; after the incubation, the beads were washed thoroughly 3 times with lysis buffer and proteins were extracted in sample buffer by boiling for 5 minutes. Then, samples were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblots were performed.
GTPase pull-down assays
Kinase assay
32D cells were transduced with retrovirus vectors. Sorted cells were lysed in TNE buffer (10 mM Tris, 200 mM NaCl, 1 mM EDTA pH 7.4, 1% Triton X-100) and HA-tagged Pak2 was isolated using HA beads. HA-Pak2 was then incubated in phospho-buffer (50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid pH 7.5, 25 mM Nacl, 1.25 mM MgCl2, 1.25 mM MnCl2), 100 μM adenosine triphosphate, and 1 μg of recombinant dephosphorylated myelin basic protein (MBP). Reactions were incubated at 30°C for 45 minutes then terminated by boiling for 5 minutes in an equal volume of 2× SDS-PAGE sample buffer. The samples were then separated on SDS-PAGE, transferred to polyvinylidene difluoride membrane and the level of phosphorylated MBP was analyzed using phosphoserine and phosphothreonine antibodies.
Immunofluorescence
Transduced and sorted GFP+ LSK cells were starved in IMDM containing 0.5% FCS for 3 hours and seeded onto fibronectin-coated (50 µg/mL; Clontech, Mountain View, CA) chamber slides that had been blocked with 2% bovine serum albumin. Cells were allowed to attach for 1 hour then stimulated with 100 ng/mL SDF1α (R&D Systems, Minneapolis, MN) for 10 minutes at 37°C, then were fixed/permeablized and washed using the Fix/Perm kit from BD Biosciences. F-Actin was stained with Rhodamine-coupled phalloidin (Invitrogen) for 1 hour, then counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) and mounted with antifade gold (Invitrogen). The slides were curated in the dark, overnight at room temperature, then sealed with nail hardener. The images were acquired using an LSM-700 confocal microscope (Zeiss, Thornwood, NY).
Cell migration assay
Transduced and sorted GFP+ LSK cells were starved in IMDM containing 0.5% FCS for 3 hours and seeded on to fibronectin-coated (50 µg/mL; Clontech) µ-slides (Ibidi USA, Madison, WI) that had been blocked with 2% BSA. Cells were allowed to adhere for 1 hour and then SDF1α (300 ng/mL; R&D Systems) was added to induce migration. The slide was mounted onto a heated, CO2 connected motorized stage and images were acquired every 30 seconds for 1 hour using a 20× objective lens on an Eclipse Ti microscope (Nikon, Melville, NY) coupled to the UltraVIEW Vox spinning disk confocal system (Perkin Elmer, Akron, OH). Cell tracks from movies were traced by using the semiautomatic tracking tool of MetaMorph software (Molecular Devices, LLC, Sunnyvale CA). [X;Y] coordinates were generated and migration was quantified using a free chemotaxis and migration tool from the Ibidi USA website.
Apoptosis
Transduced and sorted LSK cells were washed with PBS and resuspended in 300 µL of Annexin V binding buffer (BD Biosciences). The staining with Annexin V–allophycocyanin and Sytox Blue was performed as per the directions specified by the manufacturer (BD Biosciences). All samples were analyzed by an FACS LSRII or FACS Fortessa flow cytometer (BD Biosciences).
Results
Development and validation of Pak2 vectors expressing Pak2-WT and Pak2 domain mutants
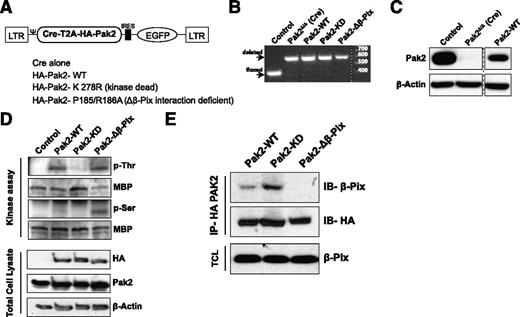
We have previously reported that Pak2 is required for HSPC homing and engraftment.9 Pak2 is a multidomain protein; to delineate the precise functions of Pak2 required for HSPC homing and engraftment, we generated retroviral vectors encoding Cre recombinase and the Pak2 full-length cDNA (Pak2–wild type [WT]) or Pak2 mutants that are defective in kinase activity (K278R, Pak2–kinase dead [KD]) or defective in binding to the guanine nucleotide exchange factor, β-Pix (P185/R186A, Pak2-Δβ-Pix) (Figure 1A). LSK cells from Pak2fl/fl mice were transduced with these vectors and DNA and protein lysates from sorted GFP+ cells were analyzed by polymerase chain reaction and immunoblot. Figure 1B demonstrates the deletion of Pak2 gene sequences while transgene expression of the Pak2-WT cDNA was confirmed by immunoblot analysis (Figure 1C) and expression of mutant cDNAs was confirmed in total cell lysates of transduced 32D cells by immunoblot for HA-tagged proteins (Figure 1D). Kinase activity of Pak2 mutant proteins was determined in an in vitro kinase assay. HA-tagged Pak proteins were immunoprecipitated and were incubated with a substrate MBP in vitro. Phosphorylation of MBP on serine or threonine residues was determined by immunoblotting. As seen in Figure 1D, both Pak2-WT and Pak2-Δβ-Pix proteins phosphorylated MBP (Figure 1D, phosphothreonine and phosphoserine bands) whereas Pak2-KD lacked this kinase activity. Defective interaction of the Pak2-Δβ-Pix mutant was confirmed by coimmunoprecipitation assay. We next confirmed that the P185/R186A (Pak2-Δβ-Pix) mutant was defective in interacting with β-Pix. Pak2-WT, Pak2-KD, or β-Pix mutants were immunoprecipitated using anti-HA antibody and the immunoprecipitates were analyzed for the presence of β-Pix protein by immunoblotting. As seen in Figure 1E, abundant levels of β-Pix protein were detected in Pak2-WT and Pak2-KD immunoprecipitates but was barely detected in Pak2-Δβ-Pix mutant. Comparable amounts of Pak2 were immunoprecipitated and equivalent amounts of β-Pix protein expressed in cell lysates confirmed the selective defect in the interaction of the Pak2-Δβ-Pix mutant with β-Pix protein.
Validation and retroviral expression of Pak2 biochemical mutants in HSPCs. (A) Schematic of polycistronic MSCV retroviral vectors expressing Cre and WT Pak2 or mutants with EGFP. (B) PCR analysis of genomic DNA extracted from FACS-sorted EGFP+ LSK cells harvested from Pak2fl/fl mice that were transduced with the indicated vectors; untransduced cells were used as a control. The positions of the WT and deleted bands are shown on the left and size ladder is shown on right. (C) Immunoblot to detect Pak2 expression in FACS-sorted EGFP+ LSK from WT (control) or Pak2fl/fl mice transduced with Cre alone (Pak2-deleted, Pak2Δ/Δ) or Cre-T2A-Pak2-WT (Pak2-WT). β-actin is used as a loading control. (D) In vitro kinase assay using MBP as a substrate and HA-tag immunoprecipitates from 32D cells transduced with vectors expressing Pak2-WT or Pak2 mutants. The blot showing phosphorylated threonine (p-Thr) and serine (p-Ser) residues and MBP indicates presence of substrate in all conditions. Bottom panel shows exogenous (HA) and total Pak2 expression from cell lysates with β-actin as a loading control. (E) Coimmunoprecipitates of HA-tagged Pak2-WT, Pak2-KD, and Pak2-Δβ-Pix from transduced 32D cell lysates analyzed by immunoblot for β-Pix. β-Pix expression in total cell lysates and HA blot of immunoprecipitates were used as loading controls. Ψ, Ψ packaging signal; EGFP, enhanced green fluorescent protein; IB, immunoblot; IP, immunoprecipitation; LTR, long terminal repeat; TCL, total cell lysate.
Validation and retroviral expression of Pak2 biochemical mutants in HSPCs. (A) Schematic of polycistronic MSCV retroviral vectors expressing Cre and WT Pak2 or mutants with EGFP. (B) PCR analysis of genomic DNA extracted from FACS-sorted EGFP+ LSK cells harvested from Pak2fl/fl mice that were transduced with the indicated vectors; untransduced cells were used as a control. The positions of the WT and deleted bands are shown on the left and size ladder is shown on right. (C) Immunoblot to detect Pak2 expression in FACS-sorted EGFP+ LSK from WT (control) or Pak2fl/fl mice transduced with Cre alone (Pak2-deleted, Pak2Δ/Δ) or Cre-T2A-Pak2-WT (Pak2-WT). β-actin is used as a loading control. (D) In vitro kinase assay using MBP as a substrate and HA-tag immunoprecipitates from 32D cells transduced with vectors expressing Pak2-WT or Pak2 mutants. The blot showing phosphorylated threonine (p-Thr) and serine (p-Ser) residues and MBP indicates presence of substrate in all conditions. Bottom panel shows exogenous (HA) and total Pak2 expression from cell lysates with β-actin as a loading control. (E) Coimmunoprecipitates of HA-tagged Pak2-WT, Pak2-KD, and Pak2-Δβ-Pix from transduced 32D cell lysates analyzed by immunoblot for β-Pix. β-Pix expression in total cell lysates and HA blot of immunoprecipitates were used as loading controls. Ψ, Ψ packaging signal; EGFP, enhanced green fluorescent protein; IB, immunoblot; IP, immunoprecipitation; LTR, long terminal repeat; TCL, total cell lysate.
Pak2 kinase activity is required for SDF1α-mediated directional migration whereas its interaction with β-Pix regulates velocity of HSPC migration
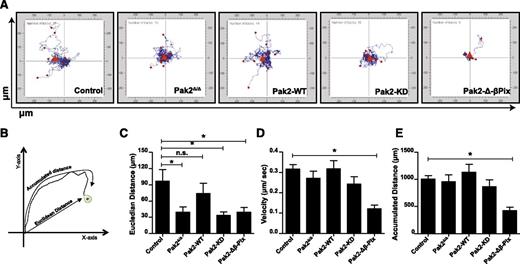
We used Pak2 biochemical mutants to dissect the role of Pak2 functional domains in directed migration. SDF1α is a potent HSC chemokine and regulates HSC homing to the BM.23-25 We tested the roles of Pak2 domains in SDF1α-induced cell migration on the extracellular matrix protein, fibronectin. Freshly isolated, transduced, and sorted LSK cells from WT (control, WT-Cre), Pak2-deleted (Pak2Δ/Δ), or Pak2-deleted cells expressing Pak2-WT or mutants were subjected to time-lapsed video microscopy in a migration assay. Individual cells were tracked for 1 hour. In Figure 2A, individual cell tracks were centered at the XY origin coordinate intersections. Cell tracks were quantified using the Metamorph image analysis program. The accumulated distance and the euclidean distance of individual cells were calculated (Figure 2B). Although the absence of Pak2 did not reduce the overall accumulated distance cells migrated, compared with control (WT), Pak2Δ/Δ HSPCs displayed reduced directional migration in response to SDF1α as quantified by the Euclidian distance moved (Figure 2C). Expression of Pak2-WT rescued the defect in directional migration (Figure 2C), whereas neither Pak2-KD nor Pak2-Δβ-Pix expression reversed this defect. Transgenic expression of Pak2-Δ-β-Pix protein led to significantly decreased total migration (Figure 2E) and reduced velocity of migration (Figure 2D), even compared with Pak2Δ/Δ cells, suggesting a possible dominant-negative effect of this protein. Taken together, these data suggest that Pak2 kinase activity and its interaction with β-Pix are required for effective SDF1α-mediated directed cell migration.
SDF1α-induced HSPC directional migration and velocity are regulated by the kinase activity of Pak2 and its interaction with β-Pix. WT and Pak2fl/fl LSK cells were transduced with indicated retroviruses, and FACS-sorted EGFP+ cells were imaged using a time-lapse microscope at 30-second intervals for 1 hour after the addition of SDF1α. The cells were tracked as described in “Methods” and analyzed using Metamorph software to generate tracks of migration for each individual cell and for quantification. (A) HSPC migration in response to SDF1α. Origins of all tracks were centered at the 0,0 XY coordinate with distance in micrometers on x- and y-axes shown (n > 11 per group). (B) Schematic representation of migration analysis; (C) the Euclidean distance in micrometers; (D) the velocity of migration in micrometers per second; and (E) the accumulated distance in micrometers. All data expressed as mean ± standard error of the mean (SEM). In panel E, only Pak2-Δβ-Pix is significantly different from control. One of the 3 independent experiments shown and the statistical significance was determined using the Mann-Whitney U test. *P < .05. n.s., not significant.
SDF1α-induced HSPC directional migration and velocity are regulated by the kinase activity of Pak2 and its interaction with β-Pix. WT and Pak2fl/fl LSK cells were transduced with indicated retroviruses, and FACS-sorted EGFP+ cells were imaged using a time-lapse microscope at 30-second intervals for 1 hour after the addition of SDF1α. The cells were tracked as described in “Methods” and analyzed using Metamorph software to generate tracks of migration for each individual cell and for quantification. (A) HSPC migration in response to SDF1α. Origins of all tracks were centered at the 0,0 XY coordinate with distance in micrometers on x- and y-axes shown (n > 11 per group). (B) Schematic representation of migration analysis; (C) the Euclidean distance in micrometers; (D) the velocity of migration in micrometers per second; and (E) the accumulated distance in micrometers. All data expressed as mean ± standard error of the mean (SEM). In panel E, only Pak2-Δβ-Pix is significantly different from control. One of the 3 independent experiments shown and the statistical significance was determined using the Mann-Whitney U test. *P < .05. n.s., not significant.
SDF1α, fibronectin-dependent F-actin remodeling, and filopodia formation require Pak2 kinase activity and its interaction with β-Pix
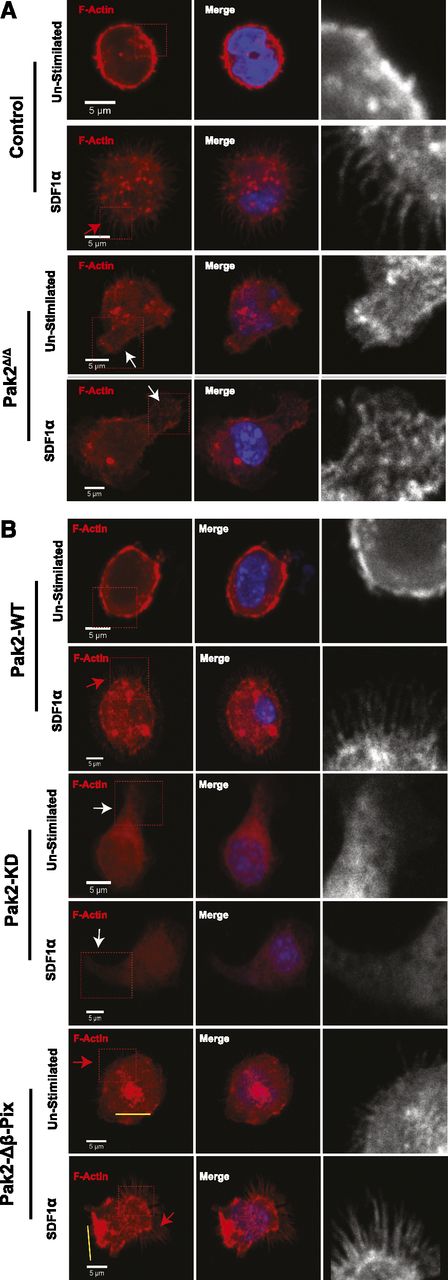
Rac and CDC42 play central roles in cytoskeletal organization. Rac regulates lamellipodia and CDC42 is a critical mediator of filopodia formation, both essential structures in directed cell migration.26-28 To further understand the chemotactic defect of Pak2Δ/Δ cells, we analyzed cytoskeletal remodeling of sorted transduced GFP+ LSK cells expressing each mutant in response to the combined agonist of SDF1α and adhesion to fibronectin. Control (WT-Cre) cells plated on fibronectin but unstimulated with SDF1α displayed relatively smooth subcortical F-actin along the cell membrane (Figure 3A inset), whereas control cells exposed to SDF1α demonstrated obvious filopodia (Figure 3A control, inset and red arrowheads). In contrast to control cells, Pak2Δ/Δ cells plated on fibronectin displayed abnormal F-actin–containing protrusions (Figure 3A, inset and white arrowheads, control vs Pak2Δ/Δ: 0.3 ± 0.3 vs 74.2 ± 0.9, percentage of cells with abnormal F-actin protrusions, n = 70-120 cells/genotype, P < .001) and failed to form filopodia in response to SDF1α (Figure 3A, Pak2Δ/Δ, control vs Pak2Δ/Δ: 56.1 ± 2.3 vs 15.1 ± 2.5, percentage of cells with filopodia, n = 70-120 cells per genotype, P < .01). Expression of Pak2-WT in Pak2-deleted cells restored smooth F-actin staining in the unstimulated condition on fibronectin and restored filopodia formation in response to SDF1α (Figure 3B, Pak2-WT, red arrowhead, Pak2Δ/Δ vs Pak2-WT: 15.1 ± 2.5 vs 62.8 ± 5.9, percentage of cells with filopodia, n = 70-120 cells per genotype, P < .01). Neither the abnormal F-actin–containing protrusions nor the filopodia formation were rescued by the expression of Pak2-KD (Figure 3B, Pak2-KD, white arrowheads, Pak2Δ/Δ vs Pak2-KD: 74.2 ± 0.8 vs 66.2 ± 7.9, percentage of cells with abnormal F-actin protrusions; 15.1 ± 2.5 vs 13.5 ± 6.7, percentage of cells with filopodia, n = 70-120 cells per genotype, P = n.s.). Expression of Pak2-Δβ-Pix restored filopodia formation (Figure 3B, Pak2-Δβ-Pix, red arrowhead, Pak2Δ/Δ vs Pak2-Δβ-Pix: 15.1 ± 2.5 vs 41.4% ± 6.5%, percentage of cells with filopodia, n = 70-120, P < .05); however, cells expressing the Pak2-Δβ-Pix mutant protein displayed abnormal F-actin bundles in both unstimulated and stimulated conditions (Figure 3B, yellow bars, control vs Pak2-Δβ-Pix: 0.4 ± 0.3 vs 56.2 ± 4.3, percentage of cells with abnormal F-actin bundles, n = 70-120, P < .01). Taken together, these data demonstrate that Pak2 interaction with β-Pix is required to restore filopodia in Pak2-deficient cells. However, both kinase activity and interaction of Pak2 with β-Pix appear required for proper F-actin remodeling and effective directional migration of HSPCs plated on fibronectin in response to SDF1α.
Pak2 regulates SDF1α- and integrin-induced filopodia formation in HSPCs in a kinase- and β-Pix–dependent manner. WT and Pak2fl/fl LSK cells were transduced with indicated retroviruses and FACS sorted. EGFP+ cells were stimulated with SDF1α and stained for F-actin using rhodamine-labeled phalloidin and counter-stained with DAPI. The slides were then imaged using Zeiss LSM 700 Laser Scanning confocal microscope with a 100× objective lens. (A) Representative images of Control (WT-Cre) and Pak2Δ/Δ. (B) Representative images of Pak2fl/fl cells transduced with Cre coexpressing Pak2-WT, Pak2-KD, and Pak2-Δβ-Pix mutants showing F-actin (red), DAPI (blue), overlap (merge). The boxed region is enlarged in grayscale in the inset to better define filopodia and abnormal protrusions containing F-actin. Red arrows indicate filopodia, white arrows indicate excessive protrusions, and yellow bars indicate abnormal F-actin bundles. The data are from 1 of 2 or more independent experiments each scoring at least 70 cells.
Pak2 regulates SDF1α- and integrin-induced filopodia formation in HSPCs in a kinase- and β-Pix–dependent manner. WT and Pak2fl/fl LSK cells were transduced with indicated retroviruses and FACS sorted. EGFP+ cells were stimulated with SDF1α and stained for F-actin using rhodamine-labeled phalloidin and counter-stained with DAPI. The slides were then imaged using Zeiss LSM 700 Laser Scanning confocal microscope with a 100× objective lens. (A) Representative images of Control (WT-Cre) and Pak2Δ/Δ. (B) Representative images of Pak2fl/fl cells transduced with Cre coexpressing Pak2-WT, Pak2-KD, and Pak2-Δβ-Pix mutants showing F-actin (red), DAPI (blue), overlap (merge). The boxed region is enlarged in grayscale in the inset to better define filopodia and abnormal protrusions containing F-actin. Red arrows indicate filopodia, white arrows indicate excessive protrusions, and yellow bars indicate abnormal F-actin bundles. The data are from 1 of 2 or more independent experiments each scoring at least 70 cells.
Pak2 regulates CDC42 activation in HSPCs
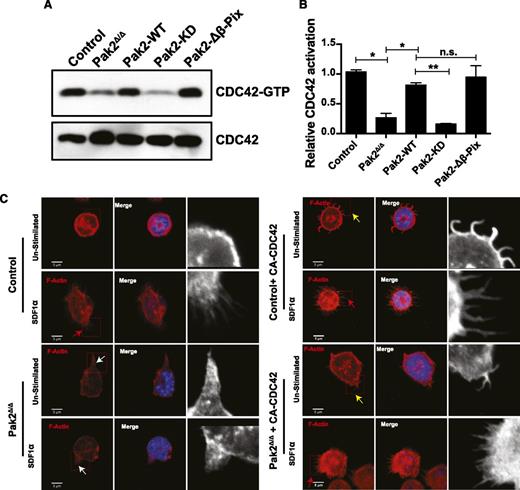
Because a major phenotype of Pak2-deficient HSPCs is defective filopodia (Figure 3A) and reduced homing,9 both phenotypes previously associated with decreased or absent CDC42 activation, we next determined whether Pak2Δ/Δ HSPCs demonstrated defective activation of CDC42. For this analysis, we performed PBD pull-down assays. As seen in Figure 4A and quantified in Figure 4B, Pak2 deletion lead to clearly reduced CDC42 activation. Expression of either Pak2-WT or Pak2-Δβ-Pix mutants, both of which maintain Pak kinase activity, restored CDC42 activation to normal levels, whereas expression of Pak2-KD did not rescue the defective CDC42 activation. These data indicate that although Pak2 has been previously considered a downstream effector of both Rac and CDC42, in HSPCs, Pak2 and specifically Pak2 kinase activity appear to regulate CDC42 activation.
Pak2 regulates CDC42 activation. (A) WT and Pak2fl/fl LSK cells were transduced with indicated retroviruses and active GTP-bound CDC42 was determined by PBD pull-down assay in the FACS-sorted EGFP+ cells in control (WT) and Pak2fl/f cells and cells coexpressing Cre and Pak2-WT, Pak2-KD, and Pak2-Δβ-Pix. CDC42 from total cell lysates was used as a loading control. (B) Normalized densitometric values comparing GTP-bound to total CDC42 from panel B. (C) Representative images of sorted SDF1-stimulated EGFP+ LSK cells from WT (control) and Pak2 fl/fl (Pak2Δ/Δ) transduced as in panel B, showing F-actin (red), DAPI (blue), overlap (merge). The boxed region is enlarged in grayscale in the inset to show filopodia/abnormal protrusions. Red arrows indicate filopodia after stimulation with SDF1α, the white arrows indicate excessive protrusions, and the yellow arrows indicate spontaneous filopodia-like formations in unstimulated conditions. Data are from 1 of 2 independent experiments each with at least 50 cells.
Pak2 regulates CDC42 activation. (A) WT and Pak2fl/fl LSK cells were transduced with indicated retroviruses and active GTP-bound CDC42 was determined by PBD pull-down assay in the FACS-sorted EGFP+ cells in control (WT) and Pak2fl/f cells and cells coexpressing Cre and Pak2-WT, Pak2-KD, and Pak2-Δβ-Pix. CDC42 from total cell lysates was used as a loading control. (B) Normalized densitometric values comparing GTP-bound to total CDC42 from panel B. (C) Representative images of sorted SDF1-stimulated EGFP+ LSK cells from WT (control) and Pak2 fl/fl (Pak2Δ/Δ) transduced as in panel B, showing F-actin (red), DAPI (blue), overlap (merge). The boxed region is enlarged in grayscale in the inset to show filopodia/abnormal protrusions. Red arrows indicate filopodia after stimulation with SDF1α, the white arrows indicate excessive protrusions, and the yellow arrows indicate spontaneous filopodia-like formations in unstimulated conditions. Data are from 1 of 2 independent experiments each with at least 50 cells.
To determine the functional consequence of Pak2 regulation of CDC42 in HSPCs, we next attempted to rescue the Pak2 cytoskeletal defects by expressing a fast-cycling, constitutively active (F28L) mutant of CDC42 (CA-CDC42) in these cells. First, expression of CA-CDC42 led to rescue of SDF1α-induced filopodia formation and attenuation of abnormal F-actin formation in Pak2Δ/Δ HSPCs (Figure 4C, Pak2Δ/Δ + CA-CDC42, red arrow, Pak2Δ/Δ vs Pak2Δ/Δ + CA-CDC42: 11.7 ± 3.3 vs 86.7 ± 0.7, percentage of cells with filopodia, n = 50, P < .01). Similar to previous reports,29 we also observed spontaneous filopodia-like extensions when CA-CDC42 was expressed in WT cells (Figure 4C inset and yellow arrowheads). Also the excessive protrusions in Pak2-deleted (Pak2Δ/Δ inset and white arrowheads) cells were corrected upon CA-CDC42 expression (Pak2Δ/Δ + CA-CDC42, Pak2Δ/Δ vs Pak2Δ/Δ + CA-CDC42: 73.6 ± 2.6 vs 13.3 ± 0.6, the percentage of cells with abnormal F-actin protrusions, n = 50, P < .01). Taken together, these data demonstrate that CA-CDC42 expression rescued Pak2 deletion–mediated deregulated cytoskeleton.
Pak2 kinase activity and its interaction with β-Pix regulate HSPC homing but Pak2 deficiency can be rescued with CA-CDC42
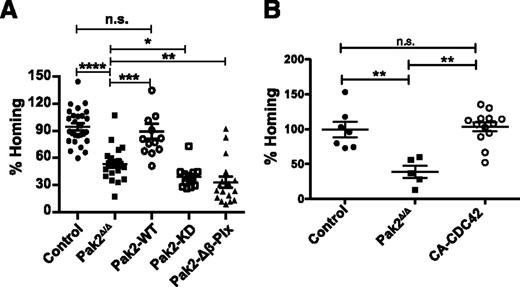
Next, to further dissect the functional roles of Pak2 domains that regulate kinase activity vs scaffolding and cytoskeletal reorganization, we examined the relative contribution of Pak2 kinase activity and its interaction with β-Pix to homing of HSPCs to the BM. We thus determined the homing efficiency of sorted transduced GFP+ LSK cells expressing each Pak2 mutant compared with Pak2-WT and Pak2Δ/Δ HSPCs. Confirming our previous data, we observed reduced BM homing of Pak2Δ/Δ HSPCs compared with control HSPCs (Figure 5A). The defective homing of Pak2Δ/Δ HSPCs was rescued by the transgenic expression of Pak2-WT (Figure 5A). Neither the expression of Pak2-KD nor Pak2-Δβ-Pix rescued the homing of Pak2Δ/Δ HSPCs. These data indicate that both Pak2 kinase activity and its interaction with β-Pix protein is required for HSPC homing to the BM. Next, we determined whether expression of CA-CDC42 could rescue the homing phenotype of Pak2Δ/Δ. As seen in Figure 5B, expression of CA-CDC42 in Pak2Δ/Δ HSPCs was associated with homing of HSPCs at levels that were comparable to Pak2-WT–corrected HSPCs (Figure 5A).
Pak2 kinase activity and its interaction with β-Pix regulate HSPC homing and CA-CDC42 rescued defective homing of Pak2 deletion. (A) Homing of LSK BM cells. WT and Pak2fl/fl LSK cells were transduced with indicated retroviruses and 2 × 105 sorted EGFP+ cells were injected into lethally irradiated mice. Sixteen hours postinfusion, homing to the BM was measured after harvesting femur, tibia, and iliac crest bones. The homing efficiency of indicated genotypes is expressed as a mean of the percentage of control (WT LSK transduced with Cre) for each mouse with mean shown as horizontal bar, n = 12-27 mice per group. (B) Sorted EGFP+ LSK cells (2 × 105) from WT (control) and Pak2fl/fl mice were transduced with vectors expressing Cre alone (Pak2Δ/Δ) or Cre and CA-CDC42 and injected into lethally irradiated mice. Sixteen hours postinfusion, the homing to the BM was measured from cells harvested from femur, tibia, and iliac crest. The homing efficiency of individual animals of the indicated genotypes is shown as a percent of control with mean (horizontal bar) ± SEM of 5 to 13 mice per group. The statistical significance was determined using the Mann-Whitney U test. ****P < .0001, ***P < .001, **P < .01, *P < .05.
Pak2 kinase activity and its interaction with β-Pix regulate HSPC homing and CA-CDC42 rescued defective homing of Pak2 deletion. (A) Homing of LSK BM cells. WT and Pak2fl/fl LSK cells were transduced with indicated retroviruses and 2 × 105 sorted EGFP+ cells were injected into lethally irradiated mice. Sixteen hours postinfusion, homing to the BM was measured after harvesting femur, tibia, and iliac crest bones. The homing efficiency of indicated genotypes is expressed as a mean of the percentage of control (WT LSK transduced with Cre) for each mouse with mean shown as horizontal bar, n = 12-27 mice per group. (B) Sorted EGFP+ LSK cells (2 × 105) from WT (control) and Pak2fl/fl mice were transduced with vectors expressing Cre alone (Pak2Δ/Δ) or Cre and CA-CDC42 and injected into lethally irradiated mice. Sixteen hours postinfusion, the homing to the BM was measured from cells harvested from femur, tibia, and iliac crest. The homing efficiency of individual animals of the indicated genotypes is shown as a percent of control with mean (horizontal bar) ± SEM of 5 to 13 mice per group. The statistical significance was determined using the Mann-Whitney U test. ****P < .0001, ***P < .001, **P < .01, *P < .05.
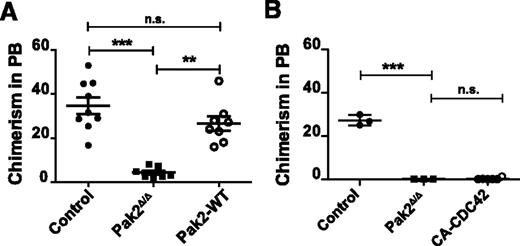
The engraftment defect of Pak2Δ/Δ HSCs can be rescued with full-length Pak2 cDNA
We next analyzed whether transgenic expression of Pak2-WT could rescue the defective engraftment of Pak2Δ/Δ HSCs. Because the Pak2fl/fl mice were not fully backcrossed, NSG mice were used as recipients for the engraftment assay. We transplanted 1 × 105 GFP+ Cre-transduced LSK cells from WT donor mice (control) and Pak2fl/fl donor mice (Pak2Δ/Δ) or Pak2fl/fl-expressing Cre and Pak2-WT cDNA (Pak2-WT) along with 5 × 105 fresh whole BM cells into sublethally (2.8 Gy) irradiated NSG mice and analyzed GFP expression in the peripheral blood 16 weeks after transplantation. Pak2Δ/Δ transplants failed to engraft recipient mice whereas this engraftment defect was rescued by the expression of Pak2-WT (Figure 6A). Interestingly, although CA-CDC42 expressed in Pak2Δ/Δ cells could rescue the homing defect, there was no rescue of the engraftment defect (Figure 6B). However, we noted that CA-CDC42 expression in WT HSCs also adversely affected engraftment (data not shown), suggesting possible toxicities of long-term CDC42 deregulation in HSCs. Thus, these data demonstrate that the regulation of CDC42 activation by Pak2 kinase activity is a key determinant of the cytoskeletal defects and homing deficiencies observed in Pak2Δ/Δ HSCs.
The engraftment defect of Pak2-deleted HSCs is rescued with full-length Pak2 cDNA. (A) Engraftment of LSK BM cells. LSK cells from WT (Control) and Pak2fl/fl mice were transduced with either Cre (Pak2Δ/Δ) or Cre-T2A-Pak2-WT (Pak2-WT) retrovirus. Sorted EGFP+ cells (1 × 105) were injected into lethally irradiated mice. Sixteen weeks posttransplantation, the percentage of EGFP-expressing cells in the peripheral blood was measured and the data plotted for each mouse with mean shown as horizontal bar, n = 7 to 11 mice per group. (B) Sorted EGFP+ LSK cells (1 × 105) from WT (control) and Pak2fl/fl (Pak2Δ/Δ) were transduced with vectors as in Figure 5B and injected into lethally irradiated mice. Sixteen weeks posttransplantation, the percentage of engrafted EGFP-expressing cells in peripheral blood was determined. The data show mean ± SEM, n = 3 to 6 mice per group. The statistical significance was determined using the Mann-Whitney U test. ***P < .001, **P < .01.
The engraftment defect of Pak2-deleted HSCs is rescued with full-length Pak2 cDNA. (A) Engraftment of LSK BM cells. LSK cells from WT (Control) and Pak2fl/fl mice were transduced with either Cre (Pak2Δ/Δ) or Cre-T2A-Pak2-WT (Pak2-WT) retrovirus. Sorted EGFP+ cells (1 × 105) were injected into lethally irradiated mice. Sixteen weeks posttransplantation, the percentage of EGFP-expressing cells in the peripheral blood was measured and the data plotted for each mouse with mean shown as horizontal bar, n = 7 to 11 mice per group. (B) Sorted EGFP+ LSK cells (1 × 105) from WT (control) and Pak2fl/fl (Pak2Δ/Δ) were transduced with vectors as in Figure 5B and injected into lethally irradiated mice. Sixteen weeks posttransplantation, the percentage of engrafted EGFP-expressing cells in peripheral blood was determined. The data show mean ± SEM, n = 3 to 6 mice per group. The statistical significance was determined using the Mann-Whitney U test. ***P < .001, **P < .01.
Discussion
In this study, we report molecular mechanisms of Pak2 in regulation of HSPC actin remodeling, migration, homing via modulation of CDC42 activation. The Rho family of small GTPases including Rac and CDC42 integrate multiple extracellular signals in HSCs to control BM homing, retention, mobilization, survival, and engraftment. Rho GTPase cycle between active, guanosine triphosphate (GTP)-bound and inactive, guanosine diphosphate–bound forms. GTP-bound GTPases regulate cellular processes through a large number of effector proteins, which are both cell and agonist specific.30 Pak kinases are well-studied downstream effectors of Rac and CDC4231 and using genetic models, Pak2 has been implicated in HSPC homing and engraftment,9 survival, proliferation, and differentiation of HSCs.32 Pak2 is a conserved serine/threonine kinase with multiple functional domains. Group A Paks, which include Pak2, contain a C-terminal kinase domain and an N-terminal regulatory domain. The regulatory domain includes a PBD and an autoinhibitory domain near a proline-rich motif mediating interactions with the GEF β-Pix.33 In previous studies, the Pix-Pak complex has been shown to target Rac1 and Pak to adhesion sites, regulating localized activation of Rac1.17 However, the specific pathways involved in Pak2 regulation of HSPC homing and engraftment have not been elucidated. In this study, we used a genetic murine model to further elucidate the role of Pak2 kinase activity and its interactions with β-Pix in HSPC actin remodeling, migration, homing, and engraftment. Unexpectedly, we also found evidence that Pak2 acts upstream of CDC42 in HSPC linking Rac and CDC42 activation and Pak2 in HSPC directional migration in response to chemokines and adhesion.
SDF1α is a selective, potent chemokine for HSCs previously shown to regulate HSC homing via the CXC chemokine receptor 4 ligation.23-25,34 Earlier studies demonstrated inhibition of group A Pak kinases (Pak1-3) in HSPCs by overexpressing Pak inhibitory peptide or genetic deletion specifically of Pak2 led to a decreased directional migration in response to SDF1α.9 Previous studies have also implicated Pak2 in thrombin-induced directional cell migration of a monocytic cell line and in formyl-methionyl-leucyl-phenylalanine–induced directional neutrophil migration.35,36 In neutrophils, Pak colocalizes with F-actin at the leading edge of migrating cells and inhibition of Pak leads to loss of cell polarization.36 The Pak2-β-Pix complex has also been reported to regulate epithelial cell migration and motility of neuronal growth cones.37,38 These studies provide functional context for the previously reported SDF1α-dependent Pak2 phosphorylation in the SEM lymphoblastic cell line.39,40 In addition, the data presented here in primary HSPCs are consistent with a recent report by Kuo et al41 who suggested, using small hairpin interfering RNA, a role for β-Pix in the migration velocity of HFF-1 fibroblasts and hypothesized that this control of cell velocity was due to β-Pix regulation of focal adhesion turnover in these cells.
Using a genetic model of Pak2 deficiency in HSPCs, we demonstrate that Pak2 interaction with β-Pix is sufficient to restore filopodia in Pak2-deficient cells, but both Pak2 kinase activity and its interaction with β-Pix appear to be required for proper F-actin remodeling and effective directional migration of HSPCs plated on fibronectin in response to SDF1α. Pak proteins have previously been implicated in both kinase-dependent and kinase-independent cytoskeletal remodeling in breast cancer and endothelial cell lines, in primary T cells, megakaryocytes, and neutrophils.19,36,42-45 In neurons, Pak regulates multiple downstream targets that influence actin-containing cell protrusions. Axon outgrowth is stimulated on laminin by partial inhibition of Pak-Pix interactions and inhibition of this complex also stimulates growth cone contacts.38 Similar to results here, Pak2 chemical inhibition in primary human neutrophils resulted in increased cell protrusions.36 These data suggest that Pak2 and β-Pix interactions in HSPCs are critical for regulating appropriate assembly and disassembly of F-actin.
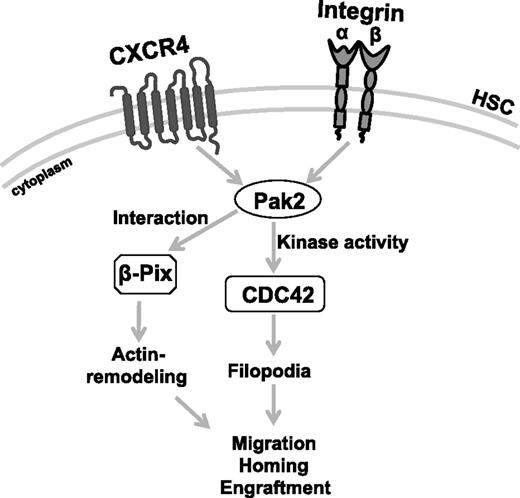
There is growing evidence of crosstalk between GTPases in a number of cells.30 The spatiotemporal relationship between activated RhoA and Rac1 is critical at the leading edge of migrating cells.46 In epithelial cells, a balance between Rho GTPases, including Rac and CDC42, appears critical for the development and maintenance of epithelial cell junctions47 and Phee et al have reported in T cells that Pak2 deletion is associated with increased activation of Rac1.43 Pak1-dependent phosphorylation of Rho–guanosine nucleotide dissociation inhibitor was shown to cause its dissociation from Rac1 and the activation of Rac1 in 293T cells.48 Here, we also demonstrate that the lack of filopodia formation in Pak2-deleted HSPCs correlated with the decreased CDC42 activation, suggesting a model in which activation of CDC42 is dependent on Pak2 and key to regulating Rac-CDC42 crosstalk in mediating HSPC homing (Figure 7). In HSPCs, the kinase activity of both Pak2-WT and Pak2-Δβ-Pix mutants restored the activation of CDC42. The importance of fine-tuned crosstalk between GTPases may be implied by our data showing CA-CDC42 could rescue homing of Pak2Δ/Δ HSPCs, whereas engraftment was only fully rescued by expression of Pak2-WT. Constitutive CDC42 activation has previously been shown to negatively affect cell survival and proliferation,49 likely due to inappropriate activation of a number of effector pathways. Although Pak2 is considered an effector protein for Rac and CDC42, the observation that abnormal activation of CDC42 was corrected in Pak2-deficient cells by expression of kinase-active Pak2 mutants is not inconsistent with previous reports implicating Pak2 upstream of Rac1.50,51 These studies contribute to the complexity of the apparent crosstalk between Rac and CDC42 in HSPCs. Given the previously identified role of both Rac and CDC42 in HSC homing,52,53 how Pak2 contributes to CDC42 activation is an important area of further study.
Model depicting Pak2 mechanism in HSPC function. Pak2 kinase activity mediates CDC42 activation to regulate filopodia formation, directional migration and its interaction with β-Pix regulates proper actin remodeling, velocity of migration to control homing, and engraftment of HSPCs in mice.
Model depicting Pak2 mechanism in HSPC function. Pak2 kinase activity mediates CDC42 activation to regulate filopodia formation, directional migration and its interaction with β-Pix regulates proper actin remodeling, velocity of migration to control homing, and engraftment of HSPCs in mice.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the members of their laboratory and Drs Leslie E. Silberstein, Jonathan H. Hartwig, Simon J. Atkinson, and Tomas Kirchhausen for the helpful discussions and advice. The authors thank Meaghan McGuinness for her assistance with mouse experiments, and Maria Suarez and Natasha Rossi for administrative assistance.
The authors acknowledge technical support from Ronald Mathieu from the Center of Molecular Development Hematopoiesis Diseases FACS and Epigenetics Technology Core and Anthony Hill from Boston Children’s Hospital Intellectual and Developmental Disabilities Research Centers Imaging Core.
This work was supported by grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases 5R01DK062757-14 (D.A.W.) and P30 DK049216-21 (Ronald Mathieu), Eunice Kennedy Shriver National Institute of Child Health and Human Development P30 HD18655 (Anthony Hill), and National Cancer Institute 5R01CA142928 (J.C.).
P.N.G.R. is supported by a fellowship from the American Society of Hematology.
Authorship
Contribution: P.N.G.R., J.C., and D.A.W. designed research; P.N.G.R., M.R., K.X., J.W., and C.E.H. performed research; P.N.G.R. and D.A.W. analyzed and interpreted the data and wrote the manuscript; and all of the authors reviewed versions of the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David A. Williams, Boston Children's Hospital, 300 Longwood Ave, Karp 08125.3, Boston, MA 02115; e-mail: dawilliams@childrens.harvard.edu.