Key Points
Single subcutaneous dosing of ACE910 has a linear PK profile, a half-life of 4 to 5 weeks, and FVIII-mimetic procoagulant activity in humans.
ACE910 at doses up to 1 mg/kg is well tolerated and has no notable adverse hypercoagulable effect in healthy Japanese and white adults.
Abstract
ACE910 is a recombinant humanized bispecific antibody that binds to activated factor IX and factor X and mimics the cofactor function of factor VIII (FVIII). This first-in-human study examined the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of ACE910 in healthy male adults. A total of 40 Japanese and 24 white subjects were randomized to receive a single subcutaneous injection of ACE910 (Japanese: 0.001, 0.01, 0.1, 0.3, or 1 mg/kg; white: 0.1, 0.3, or 1 mg/kg; n = 6 per dose group) or placebo (n = 2 per dose group). ACE910 exhibited a linear PK profile and had a half-life of ∼4 to 5 weeks. In FVIII-neutralized plasma, ACE910 shortened activated partial thromboplastin time and increased peak height of thrombin generation in a dose-dependent manner. All adverse events were nonserious and did not lead to any subject’s withdrawal. Neither clinical findings nor laboratory abnormalities indicating hypercoagulability were observed. Two of 48 subjects receiving ACE910 (1 Japanese and 1 white) were positive for anti-ACE910 antibodies (anti-drug antibodies [ADAs]). One subject tested positive for ADAs both before and after ACE910 administration, whereas the other became ADA positive after receiving ACE910. The PK and PD profiles of ACE910 were similar in healthy Japanese and white subjects and suggest that ACE910 will be an effective and convenient prophylactic treatment of hemophilia A. This trial was registered at www.clinicaltrials.jp as #JapicCTI-121934.
Introduction
Patients with severe hemophilia A (<1% residual factor VIII coagulant activity [FVIII:C]) have a much higher risk of bleeding complications than patients with moderate (1% to 5%) or mild (>5% to <40%) hemophilia A. An important goal of hemophilia A treatment is maintenance of FVIII:C ≥1%,1,2 which reduces bleeding risk, particularly at joints.3 To achieve this, intravenous recombinant or plasma-derived FVIII agents with short half-lives (8-12 hours1 ) must be administered frequently as prophylactic therapy. However, this current standard treatment of hemophilia A4 incurs a considerable physical and mental burden on patients and their families.3,5
The use of FVIII agents is complicated by interindividual variability in FVIII pharmacokinetics (PK)1,6 and requires dose or dosing frequency adjustment to maintain FVIII:C ≥1%. Further, 20% to 30% of patients with severe hemophilia A develop FVIII inhibitors (alloantibodies against FVIII) in response to therapy.1 Patients who develop FVIII inhibitors are treated with bypassing agents, including recombinant activated factor VII (rFVIIa)7 or activated prothrombin complex concentrate (aPCC).8 Frequent intravenous administration of these agents is required because of their unstable hemostatic efficacy caused by short half-lives (rFVIIa: 2.3-6.0 hours9-12 ; aPCC: 4-7 hours [thrombin generation (TG)–based half-life]13 ). New treatments with more convenient administration routes, lower administration frequency, and less immunogenicity against coagulation factors are needed.
To overcome the shortfall in the current standard of care, bispecific antibodies14 that recognize both activated factor IX (FIXa) and factor X (FX) have been developed. One of these, hBS23, demonstrated FVIII-mimetic cofactor activity in vitro in both the presence and absence of FVIII inhibitors and hemostatic activity in a nonhuman primate model of acquired hemophilia A.15 Notably, hBS23 has high subcutaneous bioavailability and a 2-week half-life in cynomolgus monkeys, suggesting that hBS23 may have a more convenient administration route with lower dosing frequency.15
Although the pharmacological concept was clearly demonstrated by hBS23, further optimization to improve FVIII-mimetic cofactor activity, PK, immunogenicity, physicochemical stability, and manufacturability resulted in ACE910, a humanized bispecific antibody with multidimensionally optimized properties.16 The hemostatic activity of ACE910 was demonstrated in a primate model of acquired hemophilia A,17 and weekly subcutaneous doses of ACE910 at 1 mg/kg in a long-term primate model significantly reduced spontaneous joint bleeds, limping, bruises, hematuria, and organ bleeds.18 Based on these preclinical results, ACE910 is expected to be a more effective and convenient prophylactic treatment of hemophilia A patients, regardless of FVIII inhibitor status.
Here, we present the first-in-human phase 1 study of ACE910, which evaluated the safety, tolerability, PK, and pharmacodynamic (PD) profiles of ACE910 in healthy adults and compared the PK and PD profiles between Japanese and white subjects.
Methods
We conducted a phase 1, first-in-human, single-center, double-blind, randomized, placebo-controlled, interindividual dose-escalation study. The study was registered at www.clinicaltrials.jp (#JapicCTI-121934), conducted at the Clinical Research Institute for Clinical Pharmacology and Therapeutics in Showa University (Tokyo, Japan) in accordance with the Declaration of Helsinki and International Conference on Harmonization–Good Clinical Practice and approved by the institutional review board. All subjects gave written informed consent before enrollment. All authors had or have access to the primary trial data.
Subjects
Healthy Japanese and white male subjects aged 20 to 44 years, with body mass index (BMI) of 18.5 to <25.0 kg/m2 (Japanese subjects) or 18.5 to <30.0 kg/m2 (white subjects), were included. Subjects with previous or current history of clinically significant allergy, hypersensitivity associated with globulin preparations, thromboembolic diseases, FVIII:C ≥120%, or abnormal protein C activity, protein S activity, antithrombin activity, lupus anticoagulant, or anti-cardiolipin-β-2 glycoprotein I complex antibody were excluded.
Study design
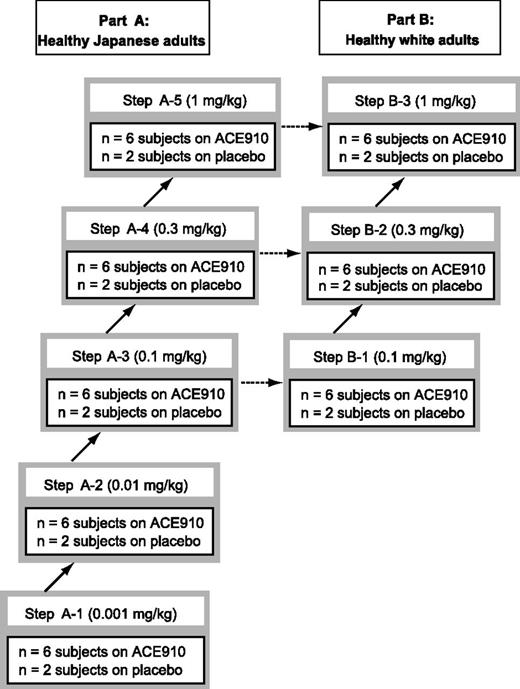
In part A, healthy Japanese subjects were randomized to receive a single subcutaneous injection of ACE910 (0.001, 0.01, 0.1, 0.3, or 1 mg/kg; n = 6 per dose group) or placebo (n = 2 per dose group) (Figure 1). The dose of 0.001 mg/kg was selected as the first-in-human dose anticipated to have minimal biological effect19 to shorten activated partial thromboplastin time (APTT) in FVIII-deficient human plasma at an ACE910 concentration of 0.01 μg/mL (data not shown). In part B, healthy white subjects were randomized to receive a single subcutaneous injection of ACE910 (0.1, 0.3, or 1 mg/kg; n = 6 per dose group) or placebo (n = 2 per dose group) (Figure 1). Subjects were observed for 4 weeks (0.001 or 0.01 mg/kg), 16 weeks (0.1 mg/kg), 20 weeks (0.3 mg/kg), or 24 weeks (1 mg/kg) until the study end. Observation periods for each dose group were based on PK considerations. Longer-term safety of a single subcutaneous injection of ACE910 was confirmed in the highest-dose group.
Study design and stepwise dose-escalation scheme. Stepwise dose-escalation scheme for healthy male Japanese subjects (part A) and white subjects (part B) is shown. A total of 40 Japanese subjects were recruited to part A, and a total of 24 white subjects were recruited to part B. Part A: Japanese subjects were randomized to receive a single subcutaneous dose of ACE910 (n = 6 for each dose group) or placebo (n = 2 for each dose group). The Japanese subjects were observed for 4 weeks at each dose, and data were reviewed prior to dose escalation. Part B: white subjects were randomized to receive a single subcutaneous injection of ACE910 (n = 6 for each dose group) or placebo (n = 2 for each dose group). The white subjects were observed for 4 weeks at each dose, and data were reviewed prior to dose escalation.
Study design and stepwise dose-escalation scheme. Stepwise dose-escalation scheme for healthy male Japanese subjects (part A) and white subjects (part B) is shown. A total of 40 Japanese subjects were recruited to part A, and a total of 24 white subjects were recruited to part B. Part A: Japanese subjects were randomized to receive a single subcutaneous dose of ACE910 (n = 6 for each dose group) or placebo (n = 2 for each dose group). The Japanese subjects were observed for 4 weeks at each dose, and data were reviewed prior to dose escalation. Part B: white subjects were randomized to receive a single subcutaneous injection of ACE910 (n = 6 for each dose group) or placebo (n = 2 for each dose group). The white subjects were observed for 4 weeks at each dose, and data were reviewed prior to dose escalation.
Administration of ACE910 or placebo followed a stepwise dose-escalation procedure (Figure 1). At each dose step in part A, ACE910 and placebo were administered to 1 subject each. After confirming vital signs, 12-lead electrocardiography (ECG) results, and adverse events (AEs) for 7 days, ACE910 or placebo was administered to the remaining 6 subjects (5 for ACE910 and 1 for placebo). Laboratory tests, vital signs, ECG results, and AEs were monitored for up to 4 weeks after administration, and PK profiles, PD responses, and serum cytokine concentrations were monitored for up to 2 weeks after administration. After tolerability and safety were confirmed, the decision to proceed to the next dose step was made. Part B was initiated after confirming the tolerability and safety of the third dose step of part A.
Drug product
ACE910 was produced from a Chinese hamster ovary cell line using recombinant DNA technology. The drug product is a solution of ACE910 at 80 mg/mL.
Outcome measures
The observation and test schedule is described in supplemental Table 1 (available on the Blood Web site).
PK
Plasma ACE910 concentrations were determined by a validated sandwich enzyme-linked immunosorbent assay. In brief, ACE910 was captured by a rabbitized anti-idiotype monoclonal antibody against FX-binding antigen-binding fragment17 and detected by a mouse anti-idiotype monoclonal antibody against FIXa-binding antigen-binding fragment,17 followed by a horseradish peroxidase–conjugated goat anti-mouse immunoglobulin G (Southern Biotechnology Associates Inc., Birmingham, AL). The lower limit of quantification was 0.05 μg/mL. Plasma concentrations of ACE910 target antigens (FIX and FX, including activated forms) were determined using commercially available sandwich enzyme-linked immunosorbent assay kits (Assaypro LLC, St. Charles, MO).
PD
APTT and peak height of TG were measured in plasma both with and without neutralization of endogenous plasma FVIII. The strategy of artificially depleting endogenous FVIII activity in plasma of healthy subjects using a dual anti-FVIII neutralizing antibody cocktail (300 μg/mL of VIII-9222 and VIII-223615 ) ex vivo was designed to mimic FVIII deficiency observed in patients with hemophilia A and to allow assessment of the PD profile of ACE910 in healthy subjects without a coagulation defect. The APTT and peak height of TG measured in plasma with neutralization of endogenous FVIII reproduced those in FVIII-deficient plasma (supplemental Figure 1).
APTT was assessed using the Thrombocheck APTT-SLA kit (Sysmex Corporation, Hyogo, Japan) and a CA-7000 (Sysmex Corporation) automated blood coagulation analyzer. Peak height of TG in plasma was measured by the calibrated automated thrombogram method using the TG fluorescence assay analysis system (Thermo Fisher Scientific, Uppsala, Sweden), with 0.47 nM human activated factor XI (Enzyme Research Laboratories, South Bend, IN) and 20 μM phospholipid as triggers.15,16 The endogenous thrombin potential was also derived from the thrombogram.
Safety
Safety was assessed by physical examination, AEs (categorized by the Medical Dictionary for Regulatory Activities code), vital signs, ECG, and laboratory tests. The severity of AEs was classified by the investigator as mild, moderate, or severe. Laboratory tests consisted of hematology tests including platelet count, blood chemistry and coagulation tests, urinalysis, and serum cytokine concentrations (interleukin [IL]-2, interferon-γ, tissue necrosis factor-α, IL-6, IL-8). Blood coagulation tests included d-dimer, thrombin-antithrombin complex (TAT), prothrombin time–international normalized ratio (PT-INR), FVIII:C, FIX activity (FIX:C), and FX activity (FX:C). FVIII:C, FIX:C, and FX:C were measured by one-stage clotting assay methods.
Immunogenicity
Anti-ACE910 antibodies (anti-drug antibodies [ADAs]) in plasma were detected by an electro-chemiluminescence immunoassay using a validated method according to US Food and Drug Administration guidance.20 For ADA-positive samples, an immunoglobulin E (IgE) antibody test was conducted using Phadia ImmunoCAP kit (Thermo Fisher Scientific Inc.). ADA-positive subjects were defined as any subject with at least 1 ADA-positive plasma sample.
Statistical analyses
Demographic, PK, PD, and safety data were summarized by race and dose, and presented as summary statistics. Data for 2 white subjects who discontinued (1 in the placebo group, 22 days after administration; 1 in the 0.3 mg/kg group, 58 days after administration) were included in the analysis.
PK parameters including maximum plasma concentration (Cmax), time to reach maximum plasma concentration (Tmax), area under the plasma concentration-time curve extrapolated to infinity (AUCinf), elimination half-life (t1/2), mean residence time (MRT), apparent total clearance (CL/F), and apparent volume of distribution (Vd/F) were calculated from each subject’s ACE910 plasma concentration-time profile by a noncompartmental analysis using WinNonlin software version 6.2 (Pharsight Corporation, St. Louis, MO). The quantitative evaluation of racial differences in PK and population PK analysis is described in supplemental Methods.
Results
Baseline characteristics
A total of 40 Japanese healthy subjects were randomized to receive a single subcutaneous injection of ACE910 (0.001, 0.01, 0.1, 0.3, or 1 mg/kg; n = 6 per group) or placebo (n = 2 corresponding to each dose group, total n = 10). A total of 24 white healthy subjects were randomized to receive a single injection of ACE910 (0.1, 0.3, or 1 mg/kg; n = 6 per group) or placebo (n = 2 per dose group, total n = 6). Within each race, the age, weight, and BMI of subjects among the ACE910 dose groups and the combined placebo group were similar (Table 1). The age and BMI of Japanese and white subjects were also similar; however, white subjects had a higher body weight.
PK
The absorption of ACE910 after subcutaneous administration was confirmed by determination of plasma ACE910 concentrations, which peaked 1 to 2 weeks after administration, with the elimination phase of the concentration-time profile appearing monophasic for both Japanese and white subjects (Figure 2). Because plasma concentrations of ACE910 at the 0.001 mg/kg dose were below the lower limit of quantification for all subjects, PK parameters were calculated for doses of 0.01 mg/kg and higher (Table 2). ACE910 showed a linear dose-exposure profile in both Japanese and white subjects, with Cmax ranging from 0.0675 to 5.92 μg/mL (0.01-1 mg/kg) and AUCinf ranging from 30.2 to 304 day × μg/mL (0.1-1 mg/kg). Notably, the half-life of ACE910 averaged 28.3 to 34.4 days, in agreement with PK data in cynomolgus monkeys, in which the half-life averaged 23.6 to 26.5 days at doses of 0.06, 0.6, and 6 mg/kg.17
Plasma ACE910 concentration after single subcutaneous injection of ACE910. The time courses of plasma ACE910 concentration after single subcutaneous injection of 0.01 mg/kg (pink reverse triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese (A) and white (B) healthy subjects are shown. Plasma ACE910 concentration was below the lower limit of quantification in all subjects at a dose of 0.001 mg/kg. Results are presented as mean ± standard deviation. Data out of the quantification range were handled as missing in summary statistics calculation. Summary statistics were not calculated when a plasma ACE910 concentration was below the lower limit of quantification in the majority of subjects at a certain dose group and time point.
Plasma ACE910 concentration after single subcutaneous injection of ACE910. The time courses of plasma ACE910 concentration after single subcutaneous injection of 0.01 mg/kg (pink reverse triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese (A) and white (B) healthy subjects are shown. Plasma ACE910 concentration was below the lower limit of quantification in all subjects at a dose of 0.001 mg/kg. Results are presented as mean ± standard deviation. Data out of the quantification range were handled as missing in summary statistics calculation. Summary statistics were not calculated when a plasma ACE910 concentration was below the lower limit of quantification in the majority of subjects at a certain dose group and time point.
The PK profiles of ACE910 in Japanese and white subjects were similar. The relative differences in Cmax and AUCinf between Japanese and white subjects were limited to 3.7% and 21.1%, respectively (supplemental Table 2).
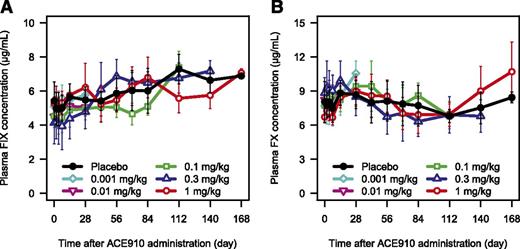
A single subcutaneous injection of ACE910 up to 1 mg/kg did not affect plasma concentrations of the target antigens, FIX and FX, in both Japanese (Figure 3; supplemental Figure 2) and white (data not shown) subjects.
Plasma FIX and FX concentrations after single subcutaneous injection of ACE910. The time courses of plasma FIX (A) and FX (B) concentration after single subcutaneous injection of placebo (black circle), 0.001 mg/kg (light blue diamond), 0.01 mg/kg (pink reverse triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese healthy subjects are shown. The concentrations of FIX and FX including their activated forms in plasma were determined. Results are presented as mean ± standard deviation.
Plasma FIX and FX concentrations after single subcutaneous injection of ACE910. The time courses of plasma FIX (A) and FX (B) concentration after single subcutaneous injection of placebo (black circle), 0.001 mg/kg (light blue diamond), 0.01 mg/kg (pink reverse triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese healthy subjects are shown. The concentrations of FIX and FX including their activated forms in plasma were determined. Results are presented as mean ± standard deviation.
PD
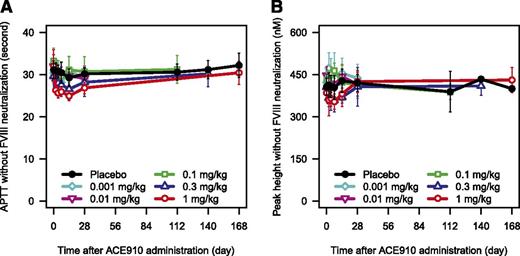
In the absence of FVIII neutralization, ACE910 slightly shortened APTT in a dose-dependent manner in both Japanese (Figure 4A; supplemental Figure 3A) and white (data not shown) subjects. Peak height of TG was not increased by ACE910 up to 1 mg/kg (Figure 4B; supplemental Figure 3B).
PD responses after single subcutaneous injection of ACE910 without neutralization of the endogenous FVIII. The time courses of APTT (A) and peak height of TG (B) after single subcutaneous injection of placebo (black circle), 0.001 mg/kg (light blue diamond), 0.01 mg/kg (pink reverse-triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese healthy subjects measured without neutralization of endogenous FVIII are shown. Results are presented as mean ± standard deviation.
PD responses after single subcutaneous injection of ACE910 without neutralization of the endogenous FVIII. The time courses of APTT (A) and peak height of TG (B) after single subcutaneous injection of placebo (black circle), 0.001 mg/kg (light blue diamond), 0.01 mg/kg (pink reverse-triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese healthy subjects measured without neutralization of endogenous FVIII are shown. Results are presented as mean ± standard deviation.
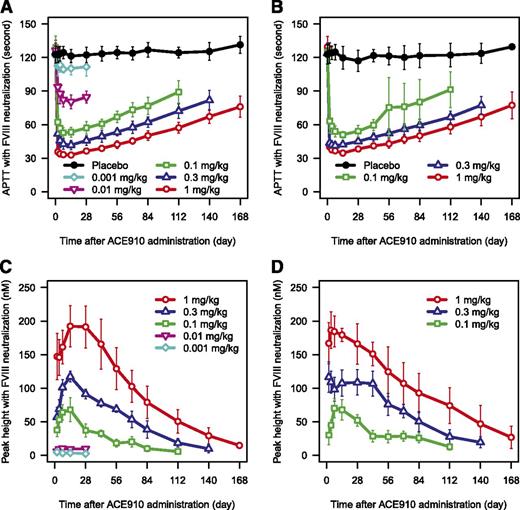
In plasma in which endogenous FVIII was neutralized ex vivo, ACE910 resulted in a dose-dependent shortening of APTT compared with baseline in both Japanese and white subjects (Figure 5A-B). An APTT similar to normal plasma was achieved at 1 mg/kg ACE910.
PD responses after single subcutaneous injection of ACE910 with neutralization of the endogenous FVIII. The time courses of APTT (A-B) and peak height of TG (C-D) after single subcutaneous injection of placebo (black circle), 0.001 mg/kg (light blue diamond), 0.01 mg/kg (pink reverse triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese (A,C) and white (B,D) healthy subjects measured with neutralization of endogenous FVIII are shown. TG was undetectable at baseline in the majority of subjects in each dose group. TG remained undetectable throughout the study period in the majority of subjects receiving placebo (data not shown). Results are presented as mean ± standard deviation. Data out of the quantification range were handled as missing in summary statistics calculation. Summary statistics were not calculated when TG was undetectable in the majority of subjects at a certain dose group and time point. One observed data point of TG was excluded because of being judged as an outlier, considering its unlikely time course and drug concentration dependency.
PD responses after single subcutaneous injection of ACE910 with neutralization of the endogenous FVIII. The time courses of APTT (A-B) and peak height of TG (C-D) after single subcutaneous injection of placebo (black circle), 0.001 mg/kg (light blue diamond), 0.01 mg/kg (pink reverse triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese (A,C) and white (B,D) healthy subjects measured with neutralization of endogenous FVIII are shown. TG was undetectable at baseline in the majority of subjects in each dose group. TG remained undetectable throughout the study period in the majority of subjects receiving placebo (data not shown). Results are presented as mean ± standard deviation. Data out of the quantification range were handled as missing in summary statistics calculation. Summary statistics were not calculated when TG was undetectable in the majority of subjects at a certain dose group and time point. One observed data point of TG was excluded because of being judged as an outlier, considering its unlikely time course and drug concentration dependency.
TG in FVIII-neutralized plasma was undetectable at baseline in most subjects in each group (data not shown). After ACE910 administration, peak height of TG increased in a dose-dependent manner in both Japanese and white subjects (Figure 5C-D). A dose-dependent increase in endogenous thrombin potential was also observed (supplemental Figure 4). Maximum peak height of TG at the maximum ACE910 dose of 1 mg/kg approached mean values of 192 nM and 186 nM for Japanese and white subjects, respectively. By comparison, baseline peak height of TG values for normal plasma without FVIII neutralization were ∼385 nM and 405 nM for Japanese and white subjects, respectively. TG remained undetectable throughout the study in most subjects receiving placebo (data not shown).
The enhanced PD response to ACE910 was observed with increased plasma ACE910 concentration (supplemental Figure 1). Accordingly, the PD response was maintained in association with the long-lasting plasma ACE910 concentration (Figure 2). The concentration-response relationship was similar for both Japanese and white subjects and reproduced in vitro data.16
Safety
ACE910 was well tolerated up to 1 mg/kg during the study (up to 24 weeks). Fifteen AEs were reported in 13 of 48 (27.1%) subjects receiving ACE910, compared with 6 AEs in 4 of 16 (25.0%) subjects receiving placebo (Table 3). All AEs were mild, except for 1 moderate AE (nasopharyngitis in 1 white subject, 0.1 mg/kg). There were no serious AEs or AEs that led to study withdrawal. Moreover, the incidence of AEs did not increase dose dependently and did not differ between Japanese and white subjects. Clinically significant changes of blood pressure, pulse rate, and body temperature were not reported. No hypersensitivity AE, serum cytokine concentration abnormality, or injection site reaction was reported following ACE910 administration.
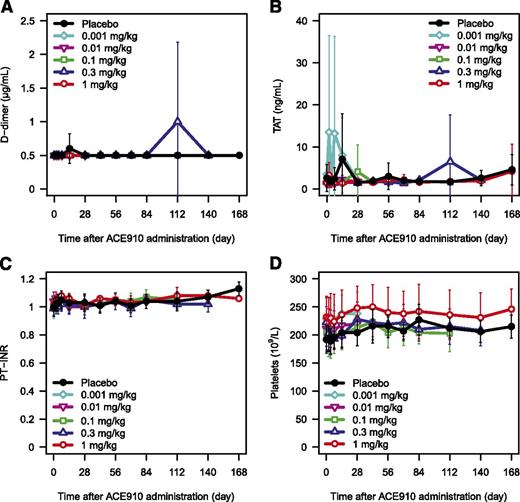
To evaluate the hypercoagulation risk of ACE910, several coagulation markers were measured. No clinically relevant abnormal coagulability resulting from ACE910 administration (from the first-in-human dose of 0.001 mg/kg to the highest dose of 1 mg/kg) was indicated by either clinical findings or laboratory tests. Laboratory values for d-dimer, TAT, PT-INR, and platelet counts did not change dose dependently in both Japanese (Figure 6A-D; supplemental Figure 5A-D) and white (data not shown) subjects. In the Japanese 0.3 mg/kg group, d-dimer and TAT transiently increased 112 days after administration in 1 subject each; both were judged by the investigator to be a blood collection artifact and not related to ACE910 because no abnormalities were observed in other coagulation markers. No clear changes in FVIII:C, FIX:C, or FX:C were observed (data not shown).
Coagulation markers and platelet count after single subcutaneous injection of ACE910. The time courses of d-dimer (A), TAT (B), PT-INR (C), and platelet count (D) after single subcutaneous injection of placebo (black circle), 0.001 mg/kg (light blue diamond), 0.01 mg/kg (pink reverse triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese healthy subjects are shown. Results are presented as mean ± standard deviation. The data out of the quantification range were imputed by the limit of quantification values.
Coagulation markers and platelet count after single subcutaneous injection of ACE910. The time courses of d-dimer (A), TAT (B), PT-INR (C), and platelet count (D) after single subcutaneous injection of placebo (black circle), 0.001 mg/kg (light blue diamond), 0.01 mg/kg (pink reverse triangle), 0.1 mg/kg (green square), 0.3 mg/kg (blue triangle), and 1 mg/kg (red circle) of ACE910 in Japanese healthy subjects are shown. Results are presented as mean ± standard deviation. The data out of the quantification range were imputed by the limit of quantification values.
Immunogenicity
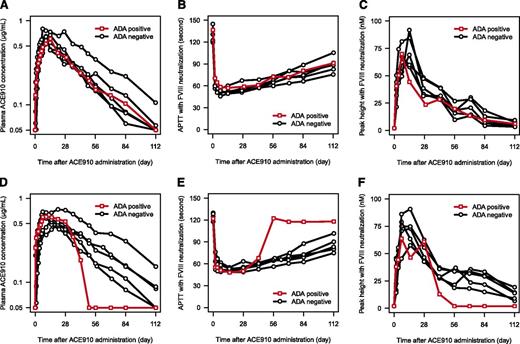
Two of 48 (4.2%) subjects receiving 0.1 mg/kg ACE910 (1 Japanese, 1 white) were ADA positive. The Japanese subject was ADA positive throughout the study, including before ACE910 administration, and had no prior treatment with antibody preparations (commercially available or investigational). Antibodies were invariably detectable at plasma dilutions of 1:160. Notably, the time course of plasma ACE910 concentration, APTT, and peak height of TG in FVIII-neutralized plasma were not different for this subject compared with those of other subjects in the same dose group (Figure 7A-C). The white subject, although initially ADA negative, tested positive 12 and 16 weeks after ACE910 administration. Antibodies were detectable at plasma dilutions of 1:1280 at 12 weeks and 1:2560 at 16 weeks. The half-life of ACE910 for this subject was relatively short at ∼9 days. Correspondingly, in FVIII-neutralized plasma, the effect of ACE910 on both APTT and peak height of TG dissipated earlier compared with ADA-negative subjects in the same dose group (Figure 7D-F). However, no significant changes in coagulation parameters, such as APTT, TG, PT-INR, FVIII:C, FIX:C, and FX:C, in plasma without FVIII neutralization were observed for this subject (data not shown). The ADA subtype was not IgE in either ADA-positive subject, and no AEs related to either abnormal coagulability or allergic reaction were observed.
Effect of anti-ACE910 antibodies on PK and PD of ACE910. The PK and PD profiles in ADA-positive subject (red open square) and ADA-negative subjects (black open circle) are shown. (A) Plasma ACE910 concentration in the Japanese 0.1 mg/kg group. (B) APTT in the Japanese 0.1 mg/kg group. (C) Peak height of TG in the Japanese 0.1 mg/kg group. (D) Plasma ACE910 concentration in the white 0.1 mg/kg group. (E) APTT in the white 0.1 mg/kg group. (F) Peak height of TG in the white 0.1 mg/kg group. The data out of the quantification range were imputed by the limit of quantification values. One observed data point of TG was excluded because of being judged as an outlier, considering its unlikely time course and drug concentration dependency.
Effect of anti-ACE910 antibodies on PK and PD of ACE910. The PK and PD profiles in ADA-positive subject (red open square) and ADA-negative subjects (black open circle) are shown. (A) Plasma ACE910 concentration in the Japanese 0.1 mg/kg group. (B) APTT in the Japanese 0.1 mg/kg group. (C) Peak height of TG in the Japanese 0.1 mg/kg group. (D) Plasma ACE910 concentration in the white 0.1 mg/kg group. (E) APTT in the white 0.1 mg/kg group. (F) Peak height of TG in the white 0.1 mg/kg group. The data out of the quantification range were imputed by the limit of quantification values. One observed data point of TG was excluded because of being judged as an outlier, considering its unlikely time course and drug concentration dependency.
Discussion
This was the first-in-human study of ACE910, a bispecific antibody that mimics FVIII cofactor function with potential application to the treatment of hemophilia A. Results demonstrated that a single subcutaneous injection of ACE910 at doses up to 1 mg/kg is well tolerated in both Japanese and white healthy male adults. In addition, the PK and PD profiles favorably support its utility as a long-acting hemostatic drug, and no obvious racial differences were observed.
Previous studies demonstrated that ACE910 has a high bioavailability (102.3%) and long half-life (23.6-26.5 days) following subcutaneous administration in cynomolgus monkeys.17 The present study confirmed that ACE910 is absorbed into plasma after subcutaneous injection in humans. Within the dose range tested, plasma ACE910 concentrations increased in a dose-proportional manner, and the elimination phase of the plasma ACE910 concentration-time profile was monophasic regardless of dose. This linear PK profile enables accurate prediction of plasma ACE910 concentration according to dose. Notably, the mean half-life of ACE910 ranged between 28.3 and 34.4 days, dramatically longer than current drugs (FVIII agents: 8-12 hours1 ; rFVIIa: 2.3-6.0 hours9-12 ; aPCC: 4-7 hours [TG-based half-life]13 ) and drugs recently approved or under development (≤19 hours).5,21-24 Concizumab is a humanized anti-tissue factor pathway inhibitor antibody and is also subcutaneously available in humans. However, because of the target-mediated drug disposition of concizumab, its half-life is shorter than ACE910.25 The apparent extended half-life of ACE910 compared with other humanized antibodies (12-20 days)26 might be a consequence of a reduced isoelectric point resulting from the amino acid–substituting antibody engineering technology applied.16,27
The current findings suggest that target-mediated drug disposition is negligible for ACE910. Target-mediated drug disposition is a nonlinear PK mechanism characteristic of antibody-based drugs.26,28,29 Binding of antibody-based drug to its target antigen is likely to increase drug clearance and decrease antigen clearance in a concentration-dependent manner. Within the dose range tested, ACE910 exhibited a linear PK profile and did not affect the clearance of FIX or FX, indicating that an immune complex is unlikely to form. This is supported by the in vitro affinities of ACE910 for FIX and FX (dissociation constants of 1.6 and 1.8 μM, respectively),30 which are much higher than the plasma concentrations of ACE910 (highest observed mean Cmax 5.92 μg/mL = 41 nM), FIX (approximate baseline mean 5.2 μg/mL = 93 nM), or FX (approximate baseline mean 7.4 μg/mL = 125 nM).
In normal plasma without FVIII neutralization, ACE910 tended to shorten APTT. However, because there were no clinical findings and laboratory tests indicative of hypercoagulability, the change was not considered a clinically relevant coagulation abnormality. Notably, when endogenous FVIII activity was depleted ex vivo by addition of anti-FVIII neutralizing antibodies, plasma samples from healthy subjects mimicked the FVIII deficiency seen in hemophilia A patients, and ACE910 shortened APTT and increased peak height of TG in a dose-dependent manner. As a consequence of the long half-life, ACE910 exhibited a long-lasting PD response throughout the study (maximum of 24 weeks). These results demonstrated the proof of pharmacology of ACE910 in humans.
Interestingly, at a dose of 1 mg/kg, APTT in FVIII-neutralized plasma reached the level equivalent to normal plasma with endogenous FVIII activity, although the peak height of TG did not. This discrepancy might be because FVIII-mimetic bispecific antibodies do not require activation to exhibit their pharmacological effect, which could result in earlier acceleration of coagulation. Therefore, the FVIII-equivalent activity of ACE910 estimated from APTT is likely to be higher than that estimated from peak height of TG.15,17,30 A previous study in a nonhuman primate model of acquired hemophilia A suggested that peak height of TG exhibits a FVIII-mimetic activity closer to reality than APTT in terms of hemostatic activity.17
To investigate the potential of ACE910 as prophylaxis in patients with hemophilia A, we simulated the plasma ACE910 concentration profile during repeated subcutaneous administration, using a population PK modeling and simulation approach (supplemental Methods; supplemental Table 3). Our simulation suggests that the plasma ACE910 concentration at steady state reaches 40 μg/mL or higher at a weekly maintenance dose of 1 mg/kg. Because of its long half-life, the predicted peak-to-trough fluctuation in plasma ACE910 concentration is small (∼5% of the difference between peak and trough levels), even with weekly dosing (supplemental Figure 6). The hemostatic potency of ACE910 at a plasma concentration of 44 μg/mL (300 nM) was assumed to be equivalent to that of 10 U/dL FVIII (10% FVIII:C) in the previous in vitro activated factor XI–triggered TG assay.16 Based on this assumption, weekly dosing of 1 mg/kg ACE910 is expected to provide constant hemostatic activity equivalent to ∼10% FVIII:C. This predicted time course of hemostatic activity is superior to current treatments for hemophilia A. In addition, the simulated plasma ACE910 concentration is higher than that demonstrated to prevent spontaneous joint bleeding in a primate model of acquired hemophilia A (∼30 μg/mL).18 These PK and PD profiles suggest that ACE910 has the potential to reduce bleeding frequency in patients with severe hemophilia A to that of patients with mild hemophilia A, even at less frequent dosing compared with existing FVIII and bypassing drugs. Furthermore, ACE910 may change the treatment paradigm from the current approach of maintaining trough levels of FVIII:C >1%1,6 to a new approach of maintaining a constant hemostatic activity corresponding to a mild hemophilia A level. On-demand use of ACE910 for bleeding events is a subject for future investigation.
ACE910 up to 1 mg/kg was well tolerated in healthy Japanese and white subjects without any serious AEs leading to study withdrawal. Importantly, although ACE910 exhibited a procoagulant effect in FVIII-neutralized plasma, markers indicative of in vivo hypercoagulability (d-dimer and TAT) did not change, even in healthy subjects with normal endogenous FVIII activity. Furthermore, there were no consumptive coagulopathy-related AEs, including PT-INR and platelet count abnormalities. Thus, ACE910 is unlikely to exhibit its procoagulant effect in vivo without coagulation triggers, which is supported by in vitro findings that the FVIII-mimetic activity of ACE910 depends on FIXa and phospholipid.15,30 These results also suggest that ACE910 exhibits an advantageous therapeutic window in patients with hemophilia A who lack endogenous FVIII activity, at an even higher dose/exposure level than that tested in this study.
The ADA-positive rate for ACE910 (2 of 48 subjects; 4.2%) was similar to that of other humanized antibodies (<1% to 10%, in most cases).29,31 Although 1 subject developed ADAs that affected both the PK and PD profiles of ACE910, there appeared to be no impact on endogenous coagulation function. Because the molecular structure of ACE910 differs from FVIII,15 ACE910 is unlikely to induce ADAs that cross-react with FVIII (ie, FVIII inhibitors). In addition, the ADAs detected in 2 subjects were not IgE, and allergic symptoms such as anaphylaxis were not observed. These findings lead to a preliminary conclusion that induction of acquired coagulation disorders, interference with the hemostatic activity of concomitant therapies, and acquisition of hypersensitivity are unlikely to occur with ACE910 therapy even if ADAs develop.
The PK and safety profiles of ACE910 were similar between Japanese and white healthy subjects, consistent with observations for other antibody-based drugs.32 Generally, racial differences in body weight and target antigen levels may contribute to the PK profile of antibody drugs.29 However, because of the body weight–adjusted dosing scheme and the negligible target-mediated drug disposition, this does not seem to occur for ACE910. This is further supported by the similar PD response observed for Japanese and white subjects. Overall, these findings indicate that dose adjustment between races is unlikely to be necessary.
Although this study presents important data pertaining to the clinical PK, PD, and safety profiles of ACE910, there are some limitations. All data were obtained from healthy subjects who received only a single subcutaneous administration up to 1 mg/kg, with an aim to minimize the potential risk of hypercoagulability when ACE910 is administered in the presence of normal FVIII activity. The accurate prediction of the prophylactic effect of ACE910 on bleeding events in hemophilia A patients, based only on PK and PD findings in healthy subjects, is limited. Further studies of the safety, tolerability, PK, PD, and efficacy of ACE910 in patients with hemophilia A are needed, and the first clinical investigation of ACE910 in patients with hemophilia A is underway (registered at www.clinicaltrials.jp as #JapicCTI-121934).
In conclusion, this first-in-human study of ACE910 demonstrated that subcutaneous injection of ACE910 at doses up to 1 mg/kg was well tolerated with a longer plasma half-life compared with currently used treatments for hemophilia A in both Japanese and white healthy male adults. The FVIII-mimetic procoagulant activity of ACE910 was successfully evaluated in a dose-dependent manner with no clear indications that ACE910 leads to hypercoagulability. The PK and PD profiles were similar between Japanese and white subjects. Based on these findings, ACE910 is expected to be a more effective prophylactic treatment of hemophilia A patients and has the potential to offer a more convenient regimen (once-weekly, subcutaneous injection) than existing therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following colleagues at Chugai Pharmaceutical Co. Ltd.: Naoki Kotani for writing support, Shingo Maisawa and Hiroko Miwa for support in the conduct of the research, and Toshihiko Aranishi for support in planning and conducting the statistical analysis. The authors also thank the subjects for their involvement in this study. The authors acknowledge the medical writing assistance provided by Ying Ke, and Rebecca Lew, CMPP, of ProScribe, part of the Envision Pharma Group (www.proscribe.com.au), funded by Chugai Pharmaceutical Co. Ltd.
This study was sponsored by Chugai Pharmaceutical Co. Ltd.
Authorship
Contribution: All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript; K.Y., N.F., T.K., and M.S. were involved in the study design; N.U., T.S., and S.K. conducted the research; and K.Y. analyzed the data and planned and conducted the statistical analysis.
Conflict-of-interest disclosure: K.Y., N.F., and T.K. are employees of Chugai Pharmaceutical Co. Ltd. M.S. received research support paid to his institution from Chugai Pharmaceutical Co. Ltd. and has received consulting honoraria from Chugai Pharmaceutical Co. Ltd. and F. Hoffmann-La Roche. K.Y. and M.S. are inventors of the patents relating to anti-FIXa/X bispecific antibodies. The remaining authors declare no competing financial interests.
Correspondence: Midori Shima, Department of Pediatrics, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8522, Japan; e-mail: mshima@naramed-u.ac.jp.