In this issue of Blood, Uchida et al report the first-in-human use of a new nonsubstitutive therapy for hemophilia A that can potentially be disruptive to the way hemophilia is treated.1
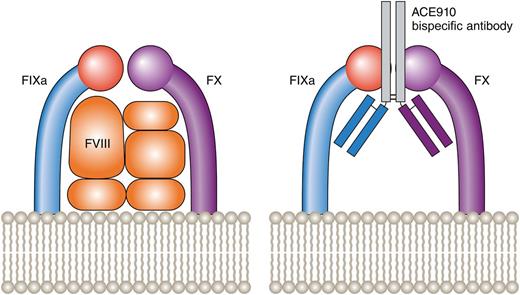
FVIII brings together FIXa and FX, allowing FXa and, ultimately, thrombin generation to proceed. The ACE910 bispecific antibody (against FIXa and FX) achieves the same action in the absence of FVIII. Professional illustration by Patrick Lane, ScEYEnce Studios.
FVIII brings together FIXa and FX, allowing FXa and, ultimately, thrombin generation to proceed. The ACE910 bispecific antibody (against FIXa and FX) achieves the same action in the absence of FVIII. Professional illustration by Patrick Lane, ScEYEnce Studios.
A disruptive technology is a new emerging technology that displaces the established one, such as what e-mail has done for personal communication or the cell phone has done to the telecommunications industry. The therapy reported bypasses factor VIII (FVIII) altogether and is a humanized bispecific immunoglobulin G antibody that juxtaposes factor IXa (FIXa) and factor X (FX) in the correct spatial arrangement allowing the generation of factor Xa (FXa).1,2 As its structure is completely distinct from FVIII, it cannot be neutralized by anti-FVIII antibodies.
The mainstay of severe and moderate hemophilia A treatment has been the infusion of plasma-derived or recombinant FVIII concentrates which have an average half-life of 8 to 12 hours.3 The recent introduction of extended half-life products has mildly increased the half-life by 1.5 to 1.7 times4 but this is unlikely to change treatment delivery in a major way. Patients who develop FVIII allo- or autoantibodies are treated with 1 of 2 bypassing agents but current treatment is IV, frequent, and less effective or predictable than FVIII concentrate use in noninhibitor patients.3 The treatment described by Uchida et al has the capacity to transform the way patients with FVIII antibodies are managed. Repeat doses are given subcutaneously at a frequency of once a week.5
FVIII is a cofactor, the function of which is to bring the enzyme FIXa in close proximity to the zymogen FX thus allowing FXa generation. ACE910, developed after screening 40 000 antibodies,2 is a bispecific antibody that was designed to replace FVIII by mediating the juxtaposition of FIXa and FX thus allowing FXa and, ultimately, thrombin generation to proceed. Studies in a nonhuman, primate model of acquired hemophilia A have previously shown that once-weekly subcutaneous ACE910 can lead to activated partial thromboplastin time (APTT) shortening, increased thrombin generation, and at least partial restoration of hemostasis.5 The Uchida et al article in this issue is the next step in the path of development of a product that is licensed for human use (see figure).
Sixty-four Japanese and white healthy volunteers were injected with increasing doses of the ACE910 antibody up to 1 mg/kg. Weekly administration of this dose is assumed to generate thrombin equivalent to FVIII coagulant activity (FVIII:C) of 0.10 IU/mL.6 There was a linear dose exposure profile without affecting the individuals’ concentration of factor IX (FIX) and FX. When the healthy participants’ FVIII was neutralized ex vivo, it was possible to demonstrate that the ACE910 was able to (in a dose-dependent manner) correct the prolonged APTT and also re-establish thrombin generation when using the calibrated automated thrombogram method.1
Phase 1 studies are designed predominantly to demonstrate safety, and no major issues were identified in this respect. The 2 complications clinicians will be most concerned about are the development of thrombosis and anti-drug antibodies (ADAs). Because this is nonsubstitutive therapy with a long half-life, thrombosis would be a potential issue if FXa generation was to continue in an uncontrolled manner. No such complication or rise in d-dimer or thrombin-antithrombin complex was observed in this small phase 1 study even though it was conducted on healthy volunteers with normal coagulation systems.
Two individuals were found to have anti-ACE910 antibodies following exposure to the bispecific antibody. One of these subjects also had antibodies before exposure to the drug but the antibody development was new in the second individual. The latter had a reduced ACE910 elimination half-life, and reduced APTT correction and thrombin generation following ex vivo FVIII neutralization, suggesting it was a functional ADA. A key issue in the long term will be the number of individuals who develop ADAs after repeated dosing with ACE910.
Although the initial aim of treatment with this antibody was as prophylaxis in patients with alloantibodies to FVIII, there is no physiological reason why this therapy would not be suitable for persons with hemophilia A and no alloantibodies, or for individuals with acquired hemophilia A and autoantibodies to FVIII. Furthermore, although ACE910 was administered once weekly in the nonhuman primate model5 because its half-life is 28.3 to 34.4 days, it is likely that future trials will investigate drug injection rates that are less frequent than this.
We are currently at a very unusual time in hemophilia treatment with a large number of new therapies in development. The majority of these are standard or extended half-life substitutive products. ACE910, however, offers the option of a disruptive technology that could potentially alter the way hemophilia care is delivered in the future.
Conflict-of-interest disclosure: The author declares no competing financial interests.