Key Points
Among evaluable patients with relapsed/refractory DLBCL who received blinatumomab 112 μg/d, overall response was 43% (CR was 19%).
Blinatumomab continuous infusion was feasible with weekly stepwise dose escalation (9-28-112 μg/d) and dexamethasone prophylaxis.
Abstract
Few patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) achieve prolonged disease-free survival. Blinatumomab, a bispecific T-cell engaging antibody construct, transiently links CD3-positive T cells to CD19-positive B cells. This phase 2 study evaluated stepwise (9-28-112 μg/d with weekly dose increases; n = 23) or flat (112 μg/d; n = 2) dosing of blinatumomab by continuous infusion, with dexamethasone prophylaxis, in patients with relapsed/refractory DLBCL. Patients received a median of 3 prior lines of therapy. Median time since last regimen was 1.5 months. Seventeen patients ended treatment in cycle 1 (induction), 7 in cycle 2 (consolidation), and 1 in retreatment. Among 21 evaluable patients, the overall response rate after 1 blinatumomab cycle was 43%, including complete responses (CRs) in 19%. Three patients had late CR in follow-up without other treatment. The most common adverse events with stepwise dosing were tremor (48%), pyrexia (44%), fatigue (26%), and edema (26%). Grade 3 neurologic events with stepwise dosing were encephalopathy and aphasia (each 9%) and tremor, speech disorder, dizziness, somnolence, and disorientation (each 4%). Of 5 (22%) patients who discontinued stepwise dosing because of adverse events, 4 (17%) had neurologic events. Most neurologic events resolved. The flat-dose cohort was stopped because of grade 3 neurologic events in both patients. Blinatumomab monotherapy appears effective in patients with relapsed/refractory DLBCL, a heavily pretreated patient population with a high unmet medical need. Further studies need to define the optimal approach to achieve the target dose without early dropout. The study was registered at www.clinicaltrials.gov as #NCT01741792.
Introduction
Outcomes of patients with diffuse large B-cell lymphoma (DLBCL) improved substantially during the past decade.1 For more than 20 years, platinum-based treatment has been considered the standard of care for patients with relapsed or refractory (r/r) DLBCL, based on response rates of 55% to 66%.2,3 For younger patients with chemosensitive relapse, consolidation with high-dose therapy and autologous hematopoietic stem cell transplant (HSCT) offers a 5-year progression-free survival (PFS) rate of ∼45%.4,5
Since the introduction of the monoclonal anti-CD20 antibody rituximab, fewer patients with DLBCL relapse, yet it is now more challenging to find effective salvage chemotherapy regimens for patients with r/r DLBCL and prior exposure to rituximab.6 Blinatumomab is a bispecific T-cell engaging (BiTE) antibody construct that transiently links CD3-positive T cells to CD19-positive B cells, inducing T-cell activation followed by serial T-cell–mediated lysis of tumor cells7-11 and concomitant T-cell proliferation.9,10 In several studies with r/r or minimal residual disease–positive acute lymphoblastic leukemia, blinatumomab was effective at doses up to 15 μg/m2 per day (28 μg/d).12-14 Blinatumomab (BLINCYTO) is approved by the US Food and Drug Administration for the treatment of Philadelphia chromosome–negative r/r B-cell precursor acute lymphoblastic leukemia.
In a phase 1 study, patients with different types of indolent and aggressive r/r B-cell non-Hodgkin lymphoma received blinatumomab in various dose schedules.15 Neurologic events were dose limiting, and the maximum tolerated dose of blinatumomab was 60 μg/m2 per day as a continuous infusion over 4 to 8 weeks. Stepwise dose escalation and corticosteroid premedication were instituted to minimize the incidence and severity of adverse events, particularly cytokine release syndrome and neurologic events. Among 35 patients treated with a weekly dose escalation schedule (5-15-60 μg/m2 per day), the overall response rate (ORR) was 69%, and the rate of complete response (CR) or unconfirmed CR was 37% across the included histologies.15,16 In a subgroup of patients with r/r DLBCL, 6 of 11 evaluable patients (55%) responded, including 4 CRs (36%), and the median response duration was 404 days (95% confidence interval [CI], 207-1129).16
In the present phase 2 study, we assessed the efficacy and safety of blinatumomab in a larger cohort of patients with r/r DLBCL and explored different blinatumomab administration regimens, including either weekly dose escalation or initiation of treatment at the target dose.
Materials and methods
Patients
The first patient was enrolled in August 2012, and the data cutoff for this primary analysis was in July 2014. Eligible patients were 18 years or older and had first or subsequent relapse of histologically confirmed DLBCL by the World Health Organization classification.17 Patients were refractory to the last treatment (defined as no response to last treatment or as relapse within 6 months from last treatment), had relapsed after autologous HSCT, or had relapsed disease and were ineligible for autologous HSCT. Other key eligibility criteria included Eastern Cooperative Oncology Group performance status ≤2, life expectancy ≥12 weeks, and adequate liver, renal, and bone marrow function. Patients with known or suspected central nervous system (CNS) involvement, history of or current relevant CNS pathologies, history of other malignancy within the last 5 years, active infection, active autoimmune disorders, or human anti-murine antibodies were excluded. Patients were excluded from study participation if they had received allogeneic HSCT at any time, autologous HSCT in the prior 6 weeks, chemotherapy in the prior 2 weeks, or radiotherapy or immunotherapy in the prior 4 weeks. Patients with transformed disease were eligible as long as histologic confirmation of DLBCL was available and the patient had received at least 1 line of treatment for aggressive lymphoma.
An independent ethics committee at each participating institution approved the study protocol and procedures. All patients provided written informed consent before enrollment. Amgen Inc was responsible for analyzing the data, and all authors had access to the primary clinical trial data.
Study design and treatment
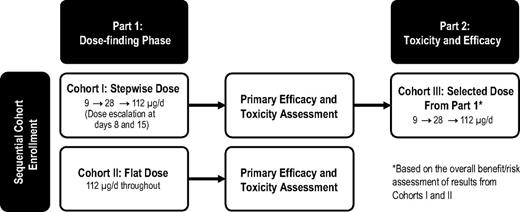
This multicenter (6 sites in Germany) open-label, single-agent phase 2 study was designed to investigate the efficacy, toxicity, and tolerability of blinatumomab in patients with r/r DLBCL, comparing stepwise dose escalation based on results of a phase 1 study of flat dosing at the target level. The study used a Simon 2-stage design18 and 2 sequential stages (Figure 1) whereby dosing in the second stage (cohort III) was selected based on the benefit/risk profile in the first stage (cohorts I and II).
Simon 2-stage study design. The study used sequential cohort enrollment whereby the dose in cohort III was based on the overall benefit/risk assessment of results from cohorts I and II.
Simon 2-stage study design. The study used sequential cohort enrollment whereby the dose in cohort III was based on the overall benefit/risk assessment of results from cohorts I and II.
The target dose in all patients was blinatumomab 112 μg/d administered by continuous IV infusion for up to 8 weeks (cycle 1), followed by 4 treatment-free weeks. Patients with CR, partial response (PR), or stable disease could receive 4 weeks of consolidation (cycle 2). The core study included cycle 1 (8 weeks), the 4-week treatment-free interval, cycle 2 (4 weeks), and a study visit 30 days after the end of infusion.
Patients in cohort I received a stepwise dose of blinatumomab, with 9 μg/d in the first week, 28 μg/d in the second week, and 112 μg/d thereafter. Patients in cohort II received a flat dose of blinatumomab (112 μg/d) starting on day 1. After cycle 1, patients entered an efficacy follow-up period with assessments every 3 months for 2 years after the first response assessment. Patients with relapse during the follow-up period could receive 8 weeks of retreatment. Administration of blinatumomab occurred on an inpatient basis at least during the first 3 days after start of infusion or dose escalation and could occur on an outpatient basis on other days.
To minimize the risk of cytokine release syndrome and neurologic events, all patients received prophylactic “early” dexamethasone for each blinatumomab infusion start and dose increase: 20 mg orally at 6 to 12 hours and at 1 hour prior to infusion, and then 8 mg orally 3 times daily for 2 days. In case of neurologic events or signs of cytokine release syndrome, dexamethasone was administered orally or by IV injection at a dose of 3 × 8 mg per day for up to 3 days, with subsequent stepwise reduction over 4 days.
Before the study, a committee was established with the responsibility of reviewing adverse events or toxicities to ensure the safety of the patients, as follows. Cohort II was started after completion of the first week of infusion of the highest target dose in the last patient of cohort I. Within cohort II, patients started infusions sequentially, with the limitation to start 1 patient per week. The Data Monitoring Committee reviewed safety data from cohort I and cohort II before the start of cohort III and assessed all grade ≥2 neurologic events in cohort II and all medically important events (eg, events leading to treatment interruption or discontinuation) in any cohort.
Statistical analysis
This report includes the primary analysis of the core study, which is now complete; the 2-year efficacy follow-up period is ongoing. The primary end point was the ORR at week 10 per independent central radiologic assessment (after 18-week course of blinatumomab) according to the Cheson revised response criteria for malignant lymphomas, including contrast-enhanced computed tomography at week 10 and fluorodeoxyglucose positron emission tomography (PET) examination at week 11.19 Key secondary end points were CR and PR rates, duration of response (DOR), PFS, overall survival, and the incidence and severity of adverse events.
Data were summarized by assigned dose (cohorts I and III combined, cohort II), and for all 3 cohorts combined. Descriptive statistics for demographic and baseline characteristics were summarized. Time-to-event variables were analyzed with the Kaplan-Meier method.
The primary analysis was the ORR for evaluable patients. An evaluable patient was defined as a patient who either received study drug at the intended target dose (112 μg/d) for at least 7 days or discontinued the study because of progressive disease.
The sample size was based on a Simon 2-stage design (Figure 1) with the following specifications: p0 = 0.15, p1 = 0.45, α = .05, and power = 0.8. For a total of 25 patients, 12 (6 each in cohorts I and II) were to be enrolled in the first stage and an additional 13 in the second stage. If 1 or none of 6 patients showed a CR or a PR in both cohorts of the first stage, then the trial was to be stopped.
Results
Patient population
A total of 25 patients were enrolled and treated with blinatumomab. Table 1 summarizes the baseline demographics and clinical characteristics of study participants. Median age was 66 years (range, 34-85 years) and 11 (44%) patients were women. At treatment start, 20 (80%) patients had Ann Arbor stage III or IV disease, 7 (28%) patients had bulky disease, and 5 (20%) patients had high risk by secondary International Prognostic Index. Sixteen (64%) patients had refractory disease at baseline. Ten (40%) patients had transformed DLBCL at the most recent assessment, 4 of whom were refractory after at least 1 regimen for DLBCL. Patients had received a median of 4 prior regimens (range, 1-8). The median time since the last treatment was 1.5 months (range, 0.2-73.1 months) for any antitumor treatment and 3.1 months (range, 0.8-73.1 months) for rituximab. Seven (28%) patients had received high-dose therapy followed by autologous HSCT.
Table 2 summarizes patient disposition. The planned sample size was 6 patients each in cohorts I and II. Three additional patients were enrolled in cohort I because 1 patient had received no treatment for DLBCL after transformation and 2 patients were not evaluable for efficacy per the study criteria. Two patients were enrolled in cohort II and treated with a flat dose of blinatumomab (112 μg/d) before the enrollment in this cohort was stopped for safety reasons, as discussed later. Of the 25 patients in any cohort, 17 (68%) ended in cycle 1 (induction), 7 (28%) ended in cycle 2 (consolidation), and 1 (4%) ended in retreatment. Reasons for ending in cycle 1 were disease progression (36%), adverse event (20%), and physician decision (12%). Reasons for ending in cycle 2 were adverse event (4%) and completing the core study (24%). The median duration of exposure to blinatumomab was 46.8 days (interquartile range, 22.1-76.9 days). Among patients who ended treatment in cycle 1, the median duration of exposure to blinatumomab was 29.2 days (interquartile range, 14.0-46.8 days).
Safety and tolerability
Table 3 summarizes treatment-emergent adverse events of any grade that were reported for >15% of patients with stepwise dosing (cohorts I and III combined); results for the 2 patients who received the flat dose in cohort II are discussed later. The most commonly reported adverse events with stepwise dosing were tremor (48%), pyrexia (44%), fatigue (26%), and edema (26%). No adverse event of cytokine release syndrome or cytokine storm was reported. The most commonly reported grade ≥3 adverse events with stepwise dosing were thrombocytopenia (17%) and leukopenia (17%). Table 3 also summarizes neurologic events reported for >1 patient with stepwise dosing; the most commonly reported neurologic events were tremor (48%), speech disorder (17%), dizziness (13%), and encephalopathy (13%). Grade 3 neurologic events that were reported for >1 patient were encephalopathy (9%) and aphasia (9%). No patient had a grade 4 or grade 5 neurologic event. Of the 48 total neurologic events that were reported with stepwise dosing, 46 events resolved. One patient each in cohorts I and III had a neurologic event that was reported as unresolved; both events (vocal cord paralysis and facial paresis) were considered to be unrelated to blinatumomab. The median time to onset of any neurologic event was 18.0 days, and the median time to resolution was 4.5 days.
Patients with any grade 3 neurologic event interrupted study treatment for at least 3 days. Patients discontinued study treatment for any grade 3 neurologic event that did not resolve within 14 days or any grade 4 neurologic event. Of 5 (22%) patients who discontinued stepwise dosing because of adverse events, 4 (17%) had nervous system disorders (encephalopathy, somnolence, epilepsy, or aphasia) or psychiatric disorders (confusional state or disorientation). Four (17%) patients interrupted study treatment with stepwise dosing because of neurologic events, and then restarted blinatumomab therapy within 3 to 11 days after the event, including 2 of the patients who subsequently discontinued study treatment because of adverse events.
Serious adverse events were reported for 21 (91%) patients with stepwise dosing; the most commonly reported serious adverse events were device-related infection (5 patients), pneumonia (5 patients), and pyrexia (3 patients). Investigators considered serious adverse events to be related to study treatment in 8 (35%) patients with stepwise dosing. Serious treatment-related neurologic events of aphasia and encephalopathy were each reported for 2 patients; all other treatment-related serious adverse events were each reported for 1 patient with stepwise dosing. Two patients in the stepwise dosing cohorts had fatal (grade 5) adverse events (1 disease progression and 1 pneumonia); neither of the fatal adverse events was considered by the investigator to be related to study drug.
The 2 patients who received the flat dose of 112 μg/d (cohort II) experienced serious grade 3 neurologic events that were considered related to blinatumomab therapy: 1 patient had neurologic symptoms but was able to continue study treatment after interruption and achieved a PR; the other had recurrent seizures leading to discontinuation of blinatumomab associated with the appearance of DLBCL cells in the cerebrospinal fluid that were not detected while performing lumbar puncture during the screening period, suggesting occult CNS manifestation. Both patients also experienced grade 4 adverse events: 1 patient had grade 4 neutropenia considered by the investigator to be unrelated to treatment; the other had grade 4 respiratory failure considered related to treatment, as well as bone marrow toxicity that was associated with acute viral infection and considered unrelated to treatment. Further enrollment in cohort II was terminated for safety reasons upon the recommendation of the study’s independent Data Monitoring Committee.
Efficacy
Table 4 summarizes the best tumor response in cycle 1 per independent radiologic assessment. For the 21 patients who were evaluable for efficacy, defined as those who received at least 1 week of treatment at the 112 μg/d target dose (n = 17) or those who discontinued treatment earlier because of progressive disease (n = 4), the ORR was 43% (9/21 patients), including 57% in cohort I (4/7 patients, which satisfied the prespecified criterion for additional evaluation of this regimen in cohort III), 100% in cohort II (1 patient), and 31% in cohort III (4/13 patients). The CR rate in evaluable patients was 19% (4/21 patients).
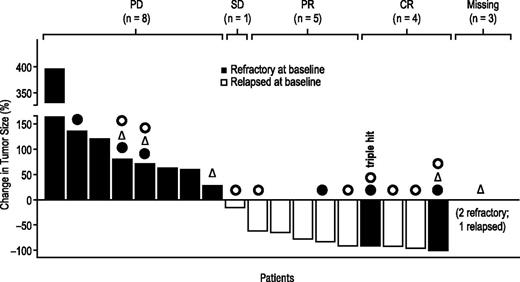
For all 25 patients who received at least 1 dose of blinatumomab, the ORR in cycle 1 was 36% overall (9/25 patients) and 35% (8/23 patients) in the stepwise dosing cohort. Figure 2 shows the change in tumor size during cycle 1 among evaluable patients.
Change in tumor size during cycle 1 (evaluable patients). Patients were evaluable if they had received study drug for at least 1 week at target dose or if study drug was stopped earlier because of progressive disease. One patient with tumor response was not evaluable per definition. “Missing” tumor size: progressive disease was assessed via clinical assessment alone in 2 patients, and 1 patient had nonmeasurable tumor at start of treatment. One patient with confirmed triple-hit lymphoma (3 gene rearrangements: c-MYC, BCL-2, and BCL-6) achieved a complete response. ● indicates patients who had previous autologous hematologic stem cell transplantation; Δ, patients with bulky disease (diameter >7.5 cm) at baseline; and ○, patients with transformed disease at baseline. PD, progressive disease; SD, stable disease.
Change in tumor size during cycle 1 (evaluable patients). Patients were evaluable if they had received study drug for at least 1 week at target dose or if study drug was stopped earlier because of progressive disease. One patient with tumor response was not evaluable per definition. “Missing” tumor size: progressive disease was assessed via clinical assessment alone in 2 patients, and 1 patient had nonmeasurable tumor at start of treatment. One patient with confirmed triple-hit lymphoma (3 gene rearrangements: c-MYC, BCL-2, and BCL-6) achieved a complete response. ● indicates patients who had previous autologous hematologic stem cell transplantation; Δ, patients with bulky disease (diameter >7.5 cm) at baseline; and ○, patients with transformed disease at baseline. PD, progressive disease; SD, stable disease.
The median DOR was 11.6 months overall (95% CI, 0.9 to not estimable). Among patients with refractory disease at baseline, the ORR was 19% (3/16 patients), and the median DOR was not reached (range, 1.9-8.8 months) because only 1 patient had a relapse recorded at the time of the analysis. Among patients with relapsed disease at baseline, the ORR was 67% (6/9 patients) and the median DOR was 8.7 months (range, 0.9-17.5 months). Supplemental Figure 1, available on the Blood Web site, shows the DOR for each of the 9 responders. Supplemental Figure 2 shows the Kaplan-Meier analysis of DOR in the responders.
For the 21 patients who were evaluable for efficacy, median PFS was 3.7 months (95% CI, 1.4-7.7), with a median follow-up of 15.0 months; the longest PFS recorded for this analysis was 20.1 months and was still ongoing at the time of the primary analysis. For all 25 patients, median overall survival was 5.0 months (95% CI, 2.3 to not estimable), with a median follow-up of 11.7 months (95% CI, 8.1-15.0) at the time of the primary analysis. Fifteen patients had died at the time of the primary analysis, including 13 patients whose death was related to disease progression (4/9 in cohort I, 1/2 in cohort II, and 8/14 in cohort III). One patient in cohort I died of cardiogenic shock 3 months after the last dose of blinatumomab (with subsequent chemotherapy) and 1 patient in cohort III died of pneumonia >1 month after the last dose of blinatumomab; neither of these deaths was considered related to blinatumomab therapy.
Three patients had a late CR in follow-up without other antilymphoma therapy. The first had an incremental late response, with a PR per central assessment after cycle 1 (induction) and a CR per investigator assessment at the 18-month follow-up visit. The second patient had a late response after slight progression, with a PR per central assessment after cycle 1 (induction) and cycle 2 (consolidation), slight progression per investigator assessment at the 3-month follow-up visit, and then a CR per investigator assessment at the 12-month follow-up visit. The third patient had a PR after cycle 1 (induction) and a CR per investigator assessment at the 3-month follow-up visit; however, the investigator subsequently observed disease progression at the 6-month follow-up visit.
Discussion
In this phase 2 study of a high-risk population with r/r DLBCL, blinatumomab monotherapy induced responses in 43% of evaluable patients and the CR rate was 19%. These phase 2 data corroborate the results from a cohort of 11 patients with DLBCL in a phase 1 study of blinatumomab. In that study, patients who received the target dose had an ORR of 55% and a CR rate of 36%.16 Long-term remissions have been observed in both the phase 1 and the phase 2 studies. The longest PFS recorded in this phase 2 study was 20.1 months and was still ongoing at the time of this primary analysis.
There is still potential for improvement in blinatumomab therapy with regard to the method of administration. In DLBCL, blinatumomab is infused continuously over 8 weeks using an ambulatory pump system. Based on experience from the phase 1 study, treatment with stepwise dose escalation over 14 days (blinatumomab at 9 μg/d in the first week, 28 μg/d in the second week, and 112 μg/d thereafter) and using an “early dexamethasone” administration before start and before every step improved tolerability in this study. But as also demonstrated in the phase 1 study and in this phase 2 study, at least 1 week of treatment at the target dose of 112 μg/d appears to be required for efficacy. Using the weekly stepwise dosing schedule in cycle 1, patients with rapidly proliferating lymphomas needed to receive blinatumomab therapy for at least 3 weeks total to receive the target dose for at least 1 week. Therefore, this study included a second cohort that started blinatumomab therapy at the 112 μg/d target dose immediately. Unfortunately, the first 2 patients in this cohort experienced grade 3 serious neurologic events during the first 72 hours. As a result, the Data Monitoring Committee recommended stopping cohort II and using the stepwise dosing in cohort III; therefore, 23 of 25 patients received the stepwise dosing schedule. In 39% of patients who received stepwise dosing, blinatumomab therapy was permanently discontinued because of early tumor progression. For these patients, achievement of an effective dose at an earlier time point with a more rapid dose-escalation schedule might have been beneficial.
In this phase 2 study, we used the 2007 revised response criteria19 for the first time in a blinatumomab study, including PET scan at week 11 (3 weeks after the end of the first cycle). Three patients who had a PR as determined by computed tomography at week 10 and confirmed by PET at week 11 achieved a CR in the follow-up period (between 3 and 12 months), suggesting either an ongoing immune response or a lag time in the demonstration of CR. This observation also suggests that the timing of PET assessment after immunotherapy still needs to be defined.
The efficacy of blinatumomab in this study is comparable to that of the novel compounds with the best published results in r/r DLBCL, including the conjugate antibodies pinatuzumab vedotin (ORR, 39%)20 and 90Y-ibrutumomab tiuxetan (ORR, 53%),21 lenalidomide (ORR, 35%),22 and the anti-PD1 antibody nivolumab (ORR, 36%).23 Lower response rates have been reported for other new drugs as monotherapy in r/r DLBCL, including inotuzumab ozogamicin (ORR, 15%),24 the Bruton tyrosine kinase inhibitor ibrutinib (ORR, 22%),25 and the phosphoinositol-3 kinase inhibitor buparlisib (ORR, 12%).26
Patients in our study were heavily pretreated, with a median of 3 prior lines of therapy. Most patients (64%) were refractory to the last treatment, as reflected by the short median time of 1.5 months since the last antitumor treatment among all patients in the study. The response rate in refractory patients was lower (ORR, 19%) than in patients with late relapses (ORR, 67%). However, all patients with discontinuation because of disease progression were refractory patients, suggesting the need for alternative strategies for early tumor control in this population.
Grade 3 neurologic events were reported for 22% of patients who received the stepwise dose and for both patients (100%) who received the flat dose. In most cases (46/48 events), the neurologic events resolved quickly after treatment discontinuation. Two neurologic events, both of which were considered to be unrelated to blinatumomab treatment, did not resolve quickly after treatment discontinuation. In the phase 1 study, which also included patients with low-grade lymphoma and different dosages, the incidence of grade 3 neurologic events was 22%.15 The possible mechanism for neurologic events is poorly understood and needs to be investigated further.
Neurologic events and cytokine release syndrome were seen with T cells transfected with the chimeric antigen receptor (CAR) directed against CD19,27,28 suggesting potential on-target effects. In contrast to blinatumomab, CAR T cells are detectable for many months after the first transfusion.29 A potential advantage of blinatumomab is that it has a short half-life and the continuous infusion can be interrupted to address some adverse events. Overall, CAR T cells and blinatumomab both have potential in the treatment of r/r DLBCL but need further improvement of the management of side effects and optimization of the treatment schedule. Future strategies for blinatumomab therapy to optimize the balance of efficacy and tolerability in r/r DLBCL may include modified dose schedules that provide the target dose earlier in the course of therapy, a better understanding of minimum treatment duration necessary to achieve responses, or using blinatumomab as consolidation therapy after initial “debulking.”
In summary, blinatumomab monotherapy may be an effective component of treatment in patients with r/r DLBCL, which is a patient population with a high unmet medical need. Stepwise dose escalation to the target dose is needed to mitigate known side effects, including neurologic events. Because r/r DLBCL progresses rapidly, an approach to blinatumomab administration that will allow patients to achieve the target dose without early dropout must be defined in further studies to ensure optimal efficacy in DLBCL.
Presented in abstract form at the 55th annual meeting (New Orleans, LA, December 2013) and the 56th annual meeting (San Francisco, CA, December 2014) of the American Society of Hematology; the 2015 annual meeting of the American Society of Clinical Oncology (Chicago, IL, June 2015); and the 13th International Conference on Malignant Lymphoma (Lugano, Switzerland, June 2015).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors sincerely thank all the patients for their participation in the study and deeply honor their dedication, and thank Geoff Smith (Amgen Inc) and Jonathan Latham (a medical writer supported by funding from Amgen Inc) for providing editorial assistance during the preparation of this manuscript.
This study was supported by research funding from Amgen Inc. The term BiTE is a registered trademark of Amgen Inc.
Authorship
Contribution: D.N. designed the research; J.S., A.Z., and D.N. analyzed the data; A.V., M.-E.G., G.H., S.N., M.P., N.A., F.Z., M.L., C.S., and R.C.B. performed the research; A.V., M.-E.G., J.S., and R.C.B. wrote the initial draft of the manuscript; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: A.V. has participated in advisory boards for Amgen Inc, Roche, Janssen, Gilead, and Bristol-Myers Squibb; received honoraria from Amgen Inc, Pfizer, and Roche; and received travel support from Roche, Pfizer, Amgen Inc, Takeda, and Janssen. M.P. has received research support from Amgen Inc and Roche and was on advisory boards for Amgen Inc, Boehringer Ingelheim, Celgene, Gilead, and Roche. M.L. has consulted for Amgen Inc. J.S. is an employee of Amgen Research (Munich). A.Z. and D.N. are employees of Amgen Inc. J.S., A.Z., and D.N. are shareholders in Amgen Inc. D.N. is an inventor on blinatumomab-related patents. R.C.B. has consulted for and received honoraria from Amgen Inc, Novartis, and GEMoaB and is an inventor of blinatumomab-related patents. The remaining authors declare no competing financial interests.
Correspondence: Andreas Viardot, Department of Internal Medicine III, University Hospital Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany; e-mail: andreas.viardot@uniklinik-ulm.de.