Inactivating mutations in the sphingosine-1-phosphate (S1P) receptor 2 (S1PR2) promoter have been associated with the germinal center (GC) B-cell diffuse large B-cell lymphoma (GCB-DLBCL) subtype. In this issue of Blood, Flori et al have now identified S1PR2 as a tumor suppressor that is transcriptionally silenced by forkhead box protein 1 (FOXP1) in the aggressive, activated B-cell (ABC-DLBCL) subtype.1
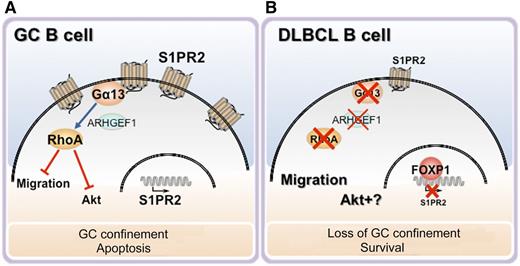
The FOXP1-S1PR2 axis in GCBs and its dysregulation in DLBCL. (A) The S1PR2/Gα13/ARFGEF1/Rho axis inhibits GCB migration and survival, resulting in their GC retention and growth control. (B) In FOXP1high DLBCL cells, FOXP1 suppresses S1PR2 expression, resulting in the loss of this inhibitory axis. Illustration by L. Patrussi, University of Siena.
The FOXP1-S1PR2 axis in GCBs and its dysregulation in DLBCL. (A) The S1PR2/Gα13/ARFGEF1/Rho axis inhibits GCB migration and survival, resulting in their GC retention and growth control. (B) In FOXP1high DLBCL cells, FOXP1 suppresses S1PR2 expression, resulting in the loss of this inhibitory axis. Illustration by L. Patrussi, University of Siena.
The lipid metabolite, S1P, has potently emerged in recent years as a central component of the complex microenvironmental cues that control lymphocyte trafficking at the systemic and local levels. This involves a tight interplay with chemokines, promoting the egress of lymphocytes that have entered secondary lymphoid organs (SLOs) from the bloodstream to return to the lymph and orchestrating their movements within lymphoid tissues to reach functionally relevant localizations. B cells, which complete their maturation in GCs following encounter with antigen, sense local S1P gradients generated by stromal cells in the S1P-poor SLO microenvironment, which allow cyclical migration to the T-cell zone to acquire help essential for affinity maturation and class switch recombination while remaining confined to GCs.2
Among the 5 known S1PRs, the most relevant in this process is S1PR2, which promotes the clustering of maturing B cells to the follicle center and limits their growth.3 This is achieved through a pathway (involving the G proteins Gα12 and Gα13, the small GTPase Rho and its activator p115RhoGEF/ARHGEF1) that suppresses the ability of B cells to respond to local chemokines that provide chemoattractant and prosurvival cues.3,4 It is therefore not surprising that alterations in S1PR2 expression and/or signaling can lead to pathogenic outcomes, as strikingly exemplified by DLBCL, the most common type of malignant B-cell lymphoma. Based on the molecular profile, DLBCL is classified into 3 major subtypes reflecting the differentiation stage of the cell of origin, of which the best characterized are the GCB and the post-GC, ABC subtypes, associated with a favorable and unfavorable prognosis, respectively. Mutations in the gene promoter resulting in S1PR2 deficiency are a distinctive feature of GCB-DLCBL.5,6 In S1PR2−/− mice, loss of GC confinement and abnormal growth and survival of B cells is associated with the development of B-cell lymphomas similar to human GCB-DLBCL.5 Taken together with the similar phenotype of mice lacking the S1PR2 signaling mediators Gα13 or p115RhoGEF,3 as well as the identification of inactivating mutations in the respective genes (GNA13 and ARHGEF1) in GCB-DLBCL,4,6 these findings strongly support the notion that S1PR2 deficiency plays a role in the pathogenesis of this neoplasm.
Remarkably, S1PR2 mutations have not been detected in ABC-DLBCL. Flori et al1 now provide evidence of an alternative mechanism of S1PR2 inactivation in ABC-DLBCL involving gene silencing by the transcriptional regulator FOXP1, which is expressed at abnormally high levels in a significant proportion of DLBCL.7 Using chromatin immunoprecipitation and gene expression profiling in initially FOXP1high DLBCL cell lines then depleted of FOXP1, Flori et al identified several genes either upregulated or downregulated by FOXP1.1 Data mining on 2 publicly accessible gene expression databases of DLBCL patients showed that, among these FOXP1 targets, only S1PR2 had a strong inverse correlation with FOXP1 expression. Importantly, when the analysis was carried out in relation to patient survival, high S1PR2 expression was found to be a positive prognostic factor, especially in combination with low FOXP1 expression. The authors also addressed the role of S1PR2 as a tumor suppressor in ABC-DLBCL, demonstrating that, when ectopically expressed in FoxP1highS1PR2low DLBCL cells, S1PR2 promotes apoptosis through a Gα13-dependent Akt-independent pathway, and limits tumor growth in subcutaneous and orthotopic disease models. They show, moreover, that loss of 1 S1PR2 allele is sufficient to accelerate tumor development in a Myc-driven B-cell lymphoma model.
The report by Flori et al provides strong mechanistic support for the implication of S1PR2 deficiency in DLBCL pathogenesis, complementing the known mutational inactivation of S1PR2 in GC-DLBCL with a new mechanism of S1PR2 silencing involving transcriptional suppression by FOXP1 in ABC-DLBCL and providing direct evidence of its role as tumor suppressor in this neoplasm.1 Additionally, the results, which link FOXP1 to changes in the levels of S1PR2 that occur during B-cell maturation in GCs, further our understanding of the regulation of S1PR2 expression, of which very little is known, not only in DLBCL B cells, but also in normal B cells. The authors show indeed that low FOXP1 correlates with high S1PR2 levels in centrocytes and centroblasts, which rely on this receptor to return to the follicle center during affinity maturation and class switch,2 whereas S1PR2 is silenced in the FOXP1high naive and memory B cells.
The results presented in this report have implications both for the treatment and for the molecular classification of ABC-DLBCL (see figure). Although the pharmacologic targeting of Akt has been proposed as a strategy to limit tumor growth in GCB-DLBCL,4 the finding by Flori et al1 that S1PR2-mediated apoptosis is Akt-dependent in DLBCL cells suggests that this approach might not be suitable for ABC-DLBCL and underscores the importance of elucidating the mechanisms linking S1PR2 deficiency to cell survival in these cells. At variance, genetic or RNAi-mediated depletion of Gα13 or p115Rho/ARHGEF1 recapitulates the effects of S1PR2 deficiency, enhancing B-cell survival even in the FoxP1highS1PR2low DLBCL cells which are not sensitive to Akt inhibition,1,4 suggesting that Rho mimetics may represent an attractive therapeutic approach for both GCB-DLBCL and ABC-DLBCL. FOXP1 and its regulators also emerge as interesting alternative targets, as FOXP1 has been implicated in the transcriptional silencing not only of S1PR2 but also of several genes that contribute to cell survival and immune surveillance in ABC-DLBCL.8,9 It is noteworthy that the prosurvival effects of FOXP1 overexpression in ABC-DLBCL rely on the nuclear factor-κB pathway,8 which is constitutively active in this disease presentation10 and for which pharmacologic inhibitors such as bortezomib are available. It will be interesting to characterize the effects of these inhibitors on the genes regulated by FOXP1, including S1PR2. Finally, the robust prognostic value of the combination of low FOXP1 with high S1PR2 expression as a positive predictor of survival in DLBCL patients under CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone) or rituximab-CHOP therapy1 may provide a new predictive biomarker for treatment stratification.
Conflict-of-interest disclosure: The author declares no competing financial interests.