In this issue of Blood, Canli et al demonstrate that reactive oxygen species (ROS) and lipid hydroperoxides can function as unconventional upstream signaling activators of receptor-interacting protein 3 (RIP3) kinase-dependent necroptosis, causing anemia in mice lacking erythroid glutathione peroxidase 4 (Gpx4).1
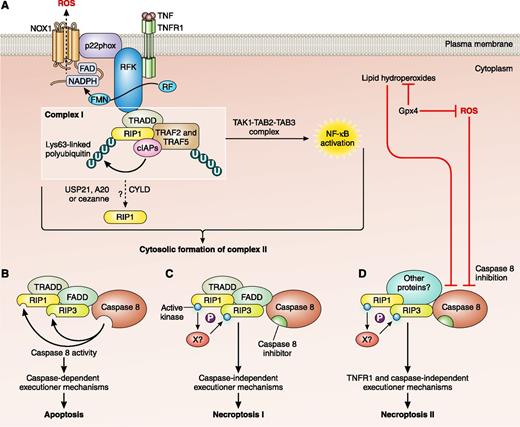
Activation pathways of cell death. (A) Binding of TNF to TNFR1 causes a conformational change and the intracellular assembly of TNFR complex I. TNFR complex I includes TRADD, RIP1, cellular inhibitor of apoptosis proteins (cIAPs), TRAF2, and TRAF5. Ubiquitylation of RIP1 results in recruitment of transforming growth factor-β–activated kinase 1 (TAK1), TAK1-binding protein 2 (TAB2), and TAB3, which initiate the nuclear factor-κB (NF-κB) activation pathway. Riboflavin kinase (RFK) links the TNFR1 death domain to p22phox, a subunit of NADPH oxidase 1 (NOX1), which contributes to TNFα-induced necroptosis by generating ROS. Deubiquitylation of RIP1 results in 2 distinct types of cell death. (B) The internalization of TNFR1 and the cytosolic assembly of TNFR complex II, which often contain TRADD, FADD, caspase 8, RIP1, and RIP3. Caspase 8 triggers apoptosis by activating the classical caspase cascade, and cleaves and inactivates RIP1 and RIP3. (C) When caspase 8 is inhibited, RIP1 and RIP3 become phosphorylated, triggering necroptosis. (D) ROS and lipid hydroperoxides are normally kept in check by Gpx4 but in oxidative conditions may increase and inhibit caspase 8. Loss of caspase 8 activity activates RIP1 and RIP3, resulting in a caspase-independent, TNFR-independent form of necroptosis. CYLD, cylindromatosis (turban tumor syndrome); FAD, flavin adenine nucleotide; FMN, flavin mononucleotide; NADPH, nicotinamide adenine dinucleotide phosphate; P, phosphate; RF, riboflavin; U, ubiquitin; USP, ubiquitin-specific peptidase; X, unidentified kinase. Adapted from Figure 1 of Vandenabeele et al3 with permission. Professional illustration by Patrick Lane, ScEYEnce Studios.
Activation pathways of cell death. (A) Binding of TNF to TNFR1 causes a conformational change and the intracellular assembly of TNFR complex I. TNFR complex I includes TRADD, RIP1, cellular inhibitor of apoptosis proteins (cIAPs), TRAF2, and TRAF5. Ubiquitylation of RIP1 results in recruitment of transforming growth factor-β–activated kinase 1 (TAK1), TAK1-binding protein 2 (TAB2), and TAB3, which initiate the nuclear factor-κB (NF-κB) activation pathway. Riboflavin kinase (RFK) links the TNFR1 death domain to p22phox, a subunit of NADPH oxidase 1 (NOX1), which contributes to TNFα-induced necroptosis by generating ROS. Deubiquitylation of RIP1 results in 2 distinct types of cell death. (B) The internalization of TNFR1 and the cytosolic assembly of TNFR complex II, which often contain TRADD, FADD, caspase 8, RIP1, and RIP3. Caspase 8 triggers apoptosis by activating the classical caspase cascade, and cleaves and inactivates RIP1 and RIP3. (C) When caspase 8 is inhibited, RIP1 and RIP3 become phosphorylated, triggering necroptosis. (D) ROS and lipid hydroperoxides are normally kept in check by Gpx4 but in oxidative conditions may increase and inhibit caspase 8. Loss of caspase 8 activity activates RIP1 and RIP3, resulting in a caspase-independent, TNFR-independent form of necroptosis. CYLD, cylindromatosis (turban tumor syndrome); FAD, flavin adenine nucleotide; FMN, flavin mononucleotide; NADPH, nicotinamide adenine dinucleotide phosphate; P, phosphate; RF, riboflavin; U, ubiquitin; USP, ubiquitin-specific peptidase; X, unidentified kinase. Adapted from Figure 1 of Vandenabeele et al3 with permission. Professional illustration by Patrick Lane, ScEYEnce Studios.
In vertebrates, the most common forms of programmed cell death are apoptosis and necroptosis, and their activation pathways overlap.2 Both may be triggered by ligation of cell death receptors, such as tumor necrosis factor (TNF) receptor 1 (TNFR1). The ligation of the receptor results in the formation of a membrane-associated complex (complex I) consisting of adaptor proteins required for downstream signaling, such as TNFR-associated death domain (TRADD) protein, TNFR-associated factor (TRAF), RIP kinases, and Fas-associated protein with death domain (FADD) (see figure, panel A). Internalization of the TNFR complex and deubiquitination of RIP1 promotes the conversion of complex I to the cytosolic complex II often comprising RIP1, RIP3, TRADD, FADD, and caspase 8. Caspase functions as a molecular switch between apoptosis and necroptosis; when caspase is inhibited the necrosome is activated.4 Activation of caspase 8 inactivates the RIP proteins and initiates apoptosis (see figure, panel B). Inhibition of caspase 8 results in activation of the RIP proteins and initiation of necroptosis (see figure, panel C). What Canli et al1 have shown is that, in erythroid cells, a different cytoplasmic necroptosis complex may be formed that is initiated by an increase in ROS and/or lipid hydroperoxides and activated by RIP3 (see figure, panel D).
During erythropoiesis, erythroid precursor cells undergo transformation, including complicated membrane and cytoskeletal rearrangements, massive protein production, accumulation of hemoglobin, and, finally, enucleation and reticulocyte maturation. This is a highly oxidative environment and the accumulation of hemoglobin in erythroid precursor cells leads to increased expression of ROS scavenging proteins and enzymes, such as catalase, peroxiredoxin 2, superoxide dismutase, glutathione peroxidase, and glutathione reductase.5 Here, Canli et al show for the first time that Gpx4 plays a key role in the homeostasis of ROS and lipid hydroperoxides in erythroid precursors. Gpx4 is essential for cell survival and embryonic development so Canli et al had to make a conditional erythroid mouse knockout.1 These Gpx4Δ mice were anemic with increased reticulocytes and increased erythroid precursor cell death. Inhibition experiments showed that the cell death did not involve TNFR or caspases. To confirm that the cell death occurred through a necroptosis pathway, the authors generated Gpx4Δ/RIP3−/− mice.1 Levels of cell death in these mice returned to normal even in the presence of high levels of ROS showing the pathway to be completely RIP3 dependent. In immunoprecipitation assays, Canli et al found that the novel necroptosis complex included RIP1, RIP3, and caspase 8 but not FADD. However, caspase 8 was inactive; absence of Gpx4 leads to functional inactivation of caspase 8 by glutathionylation. The novel necroptosis complex may also include other, as-yet-unidentified, components (see figure, panel D).
Apoptosis and necroptosis are important mechanisms for the removal of diseased and damaged cells. Autophagy, which shares some features with apoptosis, is required to remove damaged or defunct proteins from cells and plays an essential role in reticulocyte maturation.6 In contemporary culture systems for the generation of red cells the yield of mature reticulocytes is suboptimal because prolonging the cultures to maximize reticulocyte yield is accompanied by significant cell death. As the cells die, they likely release factors into the medium which exacerbate cell death. Similarly, in donor red cell storage, the cells are exposed to oxidative stress; by day 35 of refrigerated storage, up to 20% of cells may be effectively “dead” and these cells are removed by the spleen in the first 24 hours posttransfusion.7 A greater understanding of the processes of regulated erythroid cell death through necroptosis and apoptosis may increase yields and stability of manufactured red cells for transfusion therapy and may lead to improvements in the quality of stored donor red cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests.