Abstract
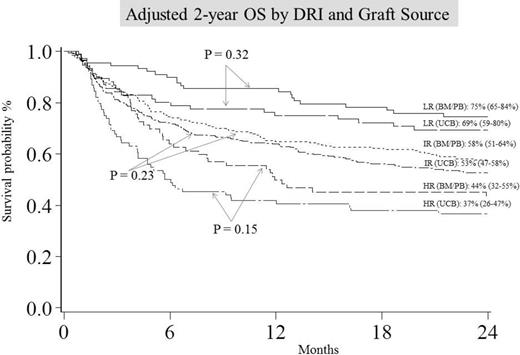
DRI incorporates both disease type and status at transplant and was found to be strong independent predictor of survival after allogeneic (allo) hematopoietic cell transplantation (HCT). Using the refined DRI assignment, we compared survival outcomes of UCB transplant (n=548: 314 with 5-6/6 and 231 with 4/6 HLA-matched units) to 8/8 HLA-matched adult donor HCT (n=528: 446 sibling and 82 unrelated; 78 bone marrow [BM] and 450 peripheral blood [PB] grafts) for hematological malignancies from 2000-2013. UCB and adult (BM/PB) groups had similar patient and transplant characteristics except UCB patients were slightly younger (47.5 year vs. 50.5 year; p <0.01), had more comorbid conditions at HCT (37.0% vs. 28.2% HCT-CI ≥3; p =0.03), and were more likely to receive reduced intensity conditioning regimen (67.7% vs. 55.9%; p <0.01). Three DRI groupings were identified: low risk (LR, 83 UCB and 110 BM/PB), intermediate risk (IR, 369 UCB and 329 PB) and high/very high risk (HR, 96 UCB and 89 BM/PB) groups. Adjusted estimates of OS at 2 years were similar with UCB or adult donors for all DRI groups (Figure). Multiple regression analysis compared outcomes of UCB with matched adult donor in each DRI group (Table). After adjusting for age and conditioning, grade III-IV acute GVHD incidence in all DRI groups was similar with both donor types. In contrast, UCB resulted in significantly lower rates of chronic GVHD in all DRI groups. Importantly, donor type was not an independent predictor of either TRM or relapse in any DRI group. In the LR-DRI group DFS was inferior with UCB, but in IR- and HR-DRI donor type had no influence on DFS after adjusting for comorbidities and CMV serostatus. Our data demonstrates that UCB is a valid alternative donor type for alloHCT, particularly those with IR and HR DRI. UCB grafts yield survival similar to matched adult donor alloHCT when DRI is considered. Low chronic GVHD risk makes UCB particularly attractive graft source for older patients.
Adjusted clinical outcomes by graft source based on DRI
| DRI Group | Graft Source | N | aGVHD Grade III-IV | cGVHD | TRM | Relapse | DFS | OS | ||||||
| HR (95%CI) | P | HR (95%CI) | P | RR (95%CI) | P | RR (95%CI) | P | HR* (95%CI) | P | HR† (95%CI) | P | |||
| Low risk | BM/PB UCB | 110 83 | 1.0 0.68 (0.36-1.26) | .82 | 1.0 0.32 (0.18-0.57) | <.01 | 1.0 1.47 (0.72-3.00) | .29 | 1.0 1.83 (0.96-3.48) | .07 | 1.0 1.70 (1.06-2.72) | .03 | 1.0 1.34 (0.75-2.41) | .32 |
| Intermediate Risk | BM/PB UCB | 329 369 | 1.0 1.01 (0.73-1.39) | .96 | 1.0 0.50 (0.38-0.67) | <.01 | 1.0 1.08 (0.76-1.52) | .66 | 1.0 0.95 (0.72-1.26) | .74 | 1.0 0.91 (0.74-1.14) | .42 | 1.0 1.17 (0.91-1.50) | .23 |
| High/very- high risk | BM/PB UCB | 89 96 | 1.0 0.75 (0.39-1.45) | .40 | 1.0 0.76 (0.60-0.96) | .02 | 1.0 1.57 (0.85-2.90) | .15 | 1.0 0.68 (0.41-.110) | .12 | 1.0 1.06 (0.73-1.54) | .76 | 1.0 1.36 (0.90-2.06) | .15 |
| DRI Group | Graft Source | N | aGVHD Grade III-IV | cGVHD | TRM | Relapse | DFS | OS | ||||||
| HR (95%CI) | P | HR (95%CI) | P | RR (95%CI) | P | RR (95%CI) | P | HR* (95%CI) | P | HR† (95%CI) | P | |||
| Low risk | BM/PB UCB | 110 83 | 1.0 0.68 (0.36-1.26) | .82 | 1.0 0.32 (0.18-0.57) | <.01 | 1.0 1.47 (0.72-3.00) | .29 | 1.0 1.83 (0.96-3.48) | .07 | 1.0 1.70 (1.06-2.72) | .03 | 1.0 1.34 (0.75-2.41) | .32 |
| Intermediate Risk | BM/PB UCB | 329 369 | 1.0 1.01 (0.73-1.39) | .96 | 1.0 0.50 (0.38-0.67) | <.01 | 1.0 1.08 (0.76-1.52) | .66 | 1.0 0.95 (0.72-1.26) | .74 | 1.0 0.91 (0.74-1.14) | .42 | 1.0 1.17 (0.91-1.50) | .23 |
| High/very- high risk | BM/PB UCB | 89 96 | 1.0 0.75 (0.39-1.45) | .40 | 1.0 0.76 (0.60-0.96) | .02 | 1.0 1.57 (0.85-2.90) | .15 | 1.0 0.68 (0.41-.110) | .12 | 1.0 1.06 (0.73-1.54) | .76 | 1.0 1.36 (0.90-2.06) | .15 |
HR indicates hazard ratio; RR, relative risk. *Denotes hazard ratio of death/relapse. †Denotes hazard ratio of death.
Bachanova:Seattle Genetics Inc.: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.