Abstract
ATG5 is a key protein that regulates autophagy, a vital cellular process whose role in various immune cells is poorly understood. A recent report showed that the deficiency of autophagy gene Atg16l1 in host DCs increased graft-vs-host disease (GVHD). Nevertheless, the direct role of autophagy in regulating T cell alloreactivity after bone marrow transplant (BMT) is unknown.
In order to investigate the role of autophagy in T cells, we first analyzed the changes in autophagosome marker LC3 upon WT T cell activation. TCR stimulation with anti-CD3/CD28, increased cytosolic LC3-I and its membrane-bound LC3-II form. Interestingly, we found that the upregulation of LC3 was predominant in dividing cells, which lead us to hypothesize that autophagy is essential for T cell proliferation. Therefore we next explored if the deficiency in autophagy impaired T cell proliferation utilizing B6 T cell-specific ATG5 knockout (ATG5 KO T cell) mouse and hydroxychloroquine (CQ, a known inhibitor of autophagy). As hypothesized, when compared with WT controls, both CQ treated WT T cells and the ATG5 KO T cells, in vitro, demonstrated a significant decrease in proliferation as demonstrated by 3H-thymidine incorporation and CFSE staining (p<0.0001) and were associated with a failure to upregulate LC3. These effects were observed after anti-CD3/CD28 TCR stimulation as well as following allogenic stimulation in a mixed lymphocyte reaction (MLR) with bone marrow-derived dendritic cells (DCs) from BALB/c mice. The reduction in T cell proliferation was accompanied by a significant increase in apoptosis (p<0.0001). However it was not associated with a decrease in T-helper (TH) signature cytokines (IFNγ, IL-2, IL-17, IL-4) suggesting no impact on T cell differentiation. Furthermore ATG5 deficiency also did not alter T cell activation as determined by upregulation of NFAT and ZAP70. Thus lack of autophagy lead to the decrease survival of T cell along with decreased proliferation after TCR stimulation but did not affect TH differentiation and T cell activation.
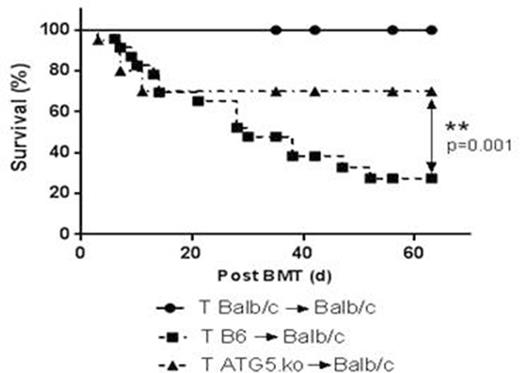
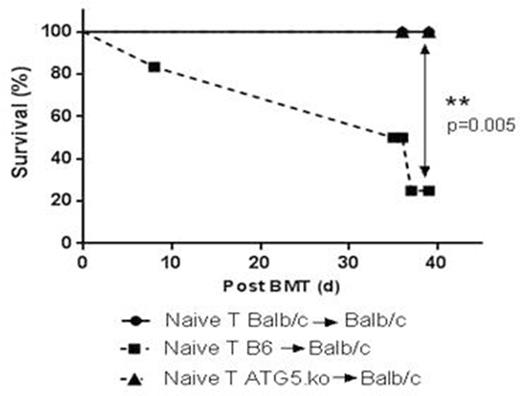
Given the in vitro observations, we hypothesized that ATG5 KO T cells would also induce less GVHD following allogenic bone marrow transplantation (BMT). Utilizing clinically relevant MHC-mismatched B6 → BALB/c BMT model, we lethally irradiated (800cGy) WT-BALB/c mice and transplanted 5x106 T cell-depleted bone marrow from WT-B6 mice along with 0.5x106 splenic T cells purified from ATG5 KO or WT- B6 mice. WT-BALB/c TCD BM and T cells were used for syngeneic controls. Consistent with in vitro results, ATG5 KO T cells showed decreased proliferation in vivo but showed no difference in Th1/Th17 differentiation. Allogenic animals transplanted with ATG5 KO T cells also showed a significantly improved survival (p=0.001) and reduced GVHD severity (p=0.03). Phenotypic analyses prior to BMT showed that ATG5 KO T cells show decreased CD62L and an increased expression of CD44. Because naïve (CD62L+CD44-) T cells are critical for GVHD, we next explored if the observed improvement in GVHD could be due to a decreased population of these naïve T cells in the transplant inoculum of ATG5 KO animals. Therefore, using the same BMT model and design, we transplanted WT-BALB/c mice 0.5x106 isolated splenic CD62L+ T cells only from either ATG5 KO or WT-B6 animals and observed that GVHD mortality was reduced in the allo-recipients of ATG5 KO T cells compared with WT T cells (p=0.005).
To determine the potential molecular mechanism, we next hypothesized that upon activation ATG KO T cells may show alterations in pro and anti-apoptotic proteins. We observed that ATG5 KO failed to increase Bcl-2 level and showed a decrease in Bcl-XL level upon TCR stimulation compared to WT T cells. In contrast to the relative lack of anti-apoptotic proteins, they displayed similar levels of the pro-apoptotic proteins BIM, Bak and Bax. These results suggest that an imbalance between pro- and anti-apoptotic factors is likely a cause for the reduced T cell expansion by ATG5 KO T cells.
Our results collectively demonstrate that inhibition of autophagy decreases T cell expansion but not its differentiation in vitro and in vivo. Furthermore contrary to the aggravation of GVHD when autophagy is targeted in host DCs, it mitigated GVHD when targeted in donor T cells. Thus the net impact of manipulating autophagy after allogeneic BMT on GVHD is dependent on which immune cell subsets are being targeted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.