Key Points
The erythroid regulator erythroferrone causes hepcidin suppression in a mouse model of thalassemia.
Ablation of Erfe in thalassemic mice restores hepcidin level to normal and decreases iron overload, but does not ameliorate anemia.
Abstract
Inherited anemias with ineffective erythropoiesis, such as β-thalassemia, manifest inappropriately low hepcidin production and consequent excessive absorption of dietary iron, leading to iron overload. Erythroferrone (ERFE) is an erythroid regulator of hepcidin synthesis and iron homeostasis. Erfe expression was highly increased in the marrow and spleen of HbbTh3/+ mice (Th3/+), a mouse model of thalassemia intermedia. Ablation of Erfe in Th3/+ mice restored normal levels of circulating hepcidin at 6 weeks of age, suggesting ERFE could be a factor suppressing hepcidin production in β-thalassemia. We examined the expression of Erfe and the consequences of its ablation in thalassemic mice from 3 to 12 weeks of age. The loss of ERFE in thalassemic mice led to full restoration of hepcidin mRNA expression at 3 and 6 weeks of age, and significant reduction in liver and spleen iron content at 6 and 12 weeks of age. Ablation of Erfe slightly ameliorated ineffective erythropoiesis, as indicated by reduced spleen index, red cell distribution width, and mean corpuscular volume, but did not improve the anemia. Thus, ERFE mediates hepcidin suppression and contributes to iron overload in a mouse model of β-thalassemia.
Introduction
Intermediate and major forms of β-thalassemia are hemoglobinopathies characterized by decreased β-globin chain synthesis or stability, microcytic anemia, ineffective erythropoiesis with elevated levels of erythropoietin (EPO), shortened red cell survival, and secondary iron overload.1 In β-thalassemia intermedia, patients do not require regular blood transfusions.1 Even without the added iron load of transfusions, pathological suppression of the iron-regulatory hormone hepcidin2-5 in patients with β-thalassemia syndromes results in excessive iron absorption. Subsequent iron accumulation in the liver, endocrine glands, and other organs leads to oxidative damage and severe clinical complications predominantly involving hepatic, endocrine, and vascular systems, but sparing the heart, at least compared with transfused patients.1 Hepcidin acts by binding to ferroportin,6 the sole known cellular iron exporter, leading to ubiquitination, internalization, and degradation of ferroportin in lysosomes.7 Decreased hepcidin production leads to the stabilization of ferroportin at the cellular membrane and promotes the absorption of dietary iron in the duodenum.
The Hbbth3/+ mouse (Th3/+) recapitulates the features of β-thalassemia intermedia in humans, including low hemoglobin levels, aberrant erythrocyte morphology, increased reticulocyte count, ineffective and extramedullary erythropoiesis, hepatosplenomegaly, and liver and spleen iron overload.8,9 In contrast to human patients who have dramatically reduced hepcidin production even in adulthood,2-5 in the Th3/+ model, liver hepcidin mRNA expression is only low in young mice compared with wild-type (WT) mice of the same age; in adult Th3/+ mice, hepcidin expression becomes similar to that of WT mice.10,11 However, even in adult Th3/+ mice, hepcidin levels are inappropriately low, considering their iron overload, consistent with the concept that inadequate levels of hepcidin cause the iron overload in thalassemic mice.
The erythroid regulator erythroferrone (ERFE) mediates hepcidin suppression during increased erythropoietic activity stimulated by endogenous or exogenous EPO, and facilitates compensatory iron acquisition during the recovery from hemorrhage-induced anemia.12 ERFE is produced by erythroid precursors in the marrow and the spleen and acts directly on the liver to decrease hepcidin production, and thereby increase iron availability for new red blood cell synthesis. We recently showed that ERFE also contributes to the recovery from anemia of inflammation by suppressing hepcidin and increasing iron availability.13 In contrast to its adaptive role in the physiological recovery from anemia, ERFE may also act pathologically to suppress hepcidin production and mediate iron overload and severe clinical complications in inherited anemias with ineffective erythropoiesis such as β-thalassemia.14 We showed that Erfe mRNA expression is highly increased in the marrow and the spleen of Th3/+ mice and that ablation of Erfe in these mice restored normal circulating levels of hepcidin and improved iron overload. The current report greatly extends these early studies.
Several studies support the concept that iron restriction may improve anemia in mouse models of thalassemia.15 Infusions of apotransferrin were shown to ameliorate anemia in a model of mild thalassemia intermedia (th1/th1).16,17 In the Th3/+ model, moderate increase in hepcidin, caused by transgenic hepcidin overexpression,8 knockdown of the negative hepcidin regulator matriptase 2,18,19 or administration of hepcidin agonists,20 not only decreased iron loading but also improved anemia.21 The hematological benefits of mild iron restriction seem to be the result of decreased α-globin precipitation, lower reactive oxygen species levels, and decreased apoptosis. Therapeutic strategies that increase hepcidin production might significantly decrease excessive iron accumulation and improve the anemia and organ damage in patients with β-thalassemia. Similar effects may be expected from interfering with overactive hepcidin-suppressive erythroid regulators, including ERFE. To determine the pathogenic contribution of ERFE in murine β-thalassemia, we studied the effect of Erfe ablation in Th3/+ mice at 3, 6, and 12 weeks of age.
Methods
Mice.
Experiments were conducted in accordance with guidelines by the National Research Council and were approved by the University of California, Los Angeles. Erfe1+/− mice on a mixed Sv129/C57BL/6 background (Fam132btm1Lex) were generated by Lexicon Pharmaceuticals22 and transferred onto a C57BL/6 background at the University of California, Los Angeles. Hbbth3/+ mice were obtained from the Jackson Laboratory (strain name B6;129P-Hbb-b1tm1UncHbb-b2tm1Unc/J) and maintained at the University of California, Los Angeles, in heterozygosity by breeding with C57BL/6 mice. HbbTh3/+ mice were mated with Erfe−/− to generate Erfe+/−, HbbTh3/+ mice, and subsequent breeding of these mice to Erfe+/− mice generated littermates of the following genotypes: WT; HbbTh3/+ (referred to as Th3/+); Erfe+/−, HbbTh3/+ (referred to as Erfe+/−, Th3/+); and Erfe−/−, HbbTh3/+ (referred to as Erfe−/−, Th3/+). Mice were maintained at the University of California, Los Angeles, on an iron-balanced diet containing 50 ppm iron (Harlan Teklad, TD 06015) and studied at the ages of 3, 6, and 12 weeks. The number of animals in each group is shown in supplemental Table 1. Preliminary analyses were performed in WT and Th3/+ littermates maintained on a standard chow (200 ppm iron; Labdiet) and analyzed at the age of 6 weeks. Three 6-week-old HbbTh3/+ mice were transfused with 400 µL packed red cells and compared with 3 untransfused controls. Red blood cells were obtained from littermate WT mice by retroorbital puncture and administered intraperitoneally after plasma removal. Control mice were given an equal volume of saline (0.9% NaCl). Six-week-old C57BL/6 male mice on standard chow were treated with a single dose of 200 U human EPO (Epogen; Amgen) and analyzed within 48 hours.
Measurement of iron and hematologic parameters.
Serum iron, spleen, and liver nonheme iron concentrations were determined as previously described,14 using acid treatment followed by a colorimetric assay for iron quantitation (Sekisui Diagnostics). Complete blood counts were obtained with a HemaVet blood analyzer (Drew Scientific). Spleen index was calculated as spleen weight (mg)/body weight (g). Enhanced Perls’ Prussian blue stain was performed as previously described.23
Quantitation of mRNA levels.
Total RNA from mouse tissues was extracted using Trizol (Invitrogen). cDNA was synthesized using iScript (Biorad). Quantitative PCR reactions were prepared with SsoAdvanced Sybr Green supermix (Biorad) and primers indicated in supplemental Table 2, and run in duplicate on a CFXconnect Instrument (Biorad). Hamp, Erfe, Gypa, Gdf15, and Twsg1 mRNA transcript abundance was normalized to the reference genes Hprt or Rpl4. Results are expressed as −ΔCt ± standard error of the mean (ie, the cycle threshold differences between reference and target genes within each group of mice).
Fluorescence-activated cell sorting.
To isolate erythroblasts at different stages of maturation, more than 15 × 106 bone marrow cells from 3 WT and 3 littermate Th3/+ mice at 12 weeks of age were sorted as previously described.12
Isolation of marrow-derived macrophages.
Bone marrow–derived macrophages were isolated from WT mouse femurs after red blood cells lysis and grown in DMEM with 10% heat-inactivated fetal calf serum and 20% L929 cell-conditioned medium for 7 days. Cells were then treated with 10 U/mL human EPO (Epogen; Amgen) for 15 hours.
ERFE enzyme-linked immunosorbent assay.
Mouse ERFE monoclonal antibodies and recombinant mouse ERFE standard were produced by Silarus Therapeutics. High binding 96-well EIA plates (Corning) were coated overnight at 4°C with 100 μL/well of 1.0 μg/mL capture antibody in 50 mM sodium carbonate buffer (pH 9.6). Plates were washed (TBS, 0.5% Tween-20) and blocked for 45 minutes with 200 μL/well SuperBlock T20 blocking buffer (Pierce). Samples and standards diluted in SuperBlock T20 were incubated for 2 hours at room temperature. Plates were washed and incubated for 1 hour with 100 μL/well of 1.0 μg/mL biotinylated detection antibody, and then washed and then incubated for 45 minutes with Streptavidin-HRP conjugate (Invitrogen). Plates were developed with 100 μL/well Supersensitive TMB substrate (Sigma) in the dark at room temperature, the reaction was stopped by adding 100 μL of 0.5 M sulfuric acid, and the absorbance was measured at 450 nm. Erfe−/− and Erfe−/−, Th3/+ were used as negative control to determine the limit of detection (100 pg/mL).
Statistical analysis.
The statistical significance of differences between groups was evaluated using the Sigmaplot 11.0 package (Systat Software). The Student t test was used to compare 2 groups of normally distributed data. The Mann-Whitney rank-sum test was used to compare data that were not normally distributed. A P value < 0.05 in a 2-tailed test was considered statistically significant.
Results
Expression profiling of ERFE in thalassemic mice.
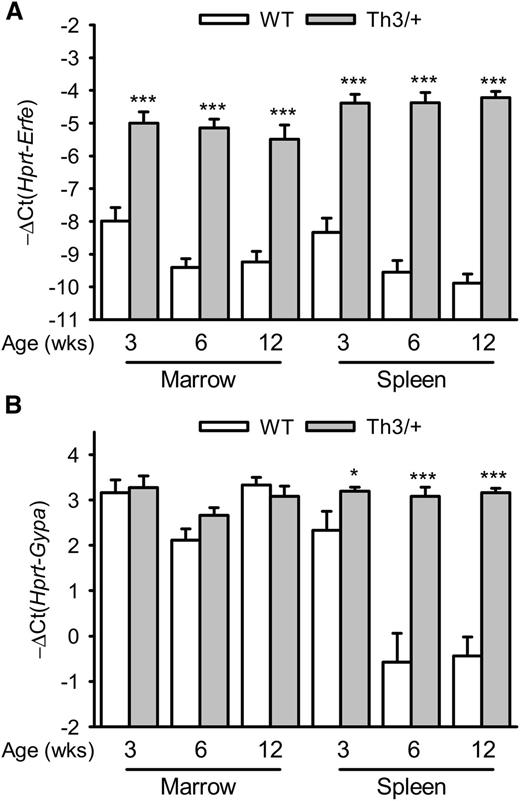
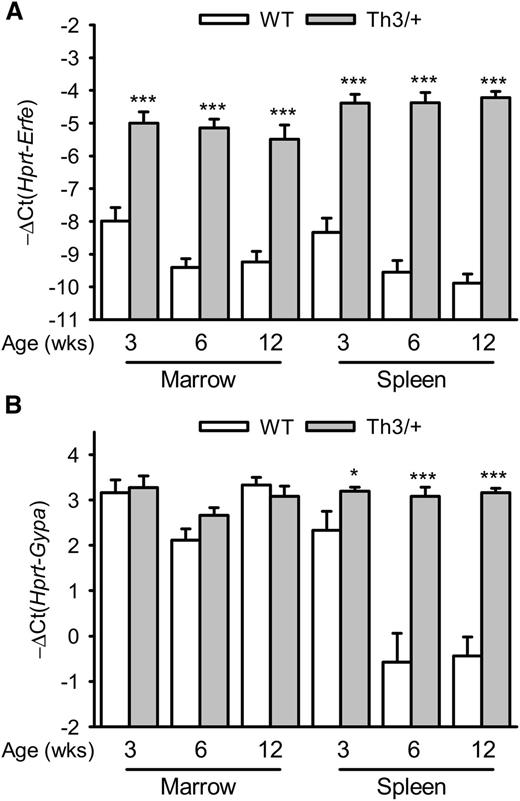
We first assessed the expression of Erfe (Fam132b) mRNA in the marrow and spleen of Th3/+ mice compared with WT mice at 3, 6, and 12 weeks of age. Erfe mRNA levels were increased 8- to 32-fold in the marrow and 16- to 32-fold in the spleen of 3- to 12-week-old Th3/+ mice compared with WT mice and had already reached their maximal expression level at 3 weeks of age (Figure 1A). Consistent with a time of rapid growth and erythroid expansion, Erfe mRNA levels were higher in 3-week-old WT mice compared with 6- and 12-week-old animals in the marrow (P = .02 and .014) and in the spleen (P = .042 and .005, respectively). In the spleen, glycophorin A (Gypa) mRNA expression was higher in Th3/+ mice compared with WT mice, reflecting extramedullary erythropoiesis (Figure 1B). To discern the relative contributions of changes in erythroid precursor numbers and changes in Erfe mRNA expression per erythroid cell, we analyzed Erfe expression relative to Gypa (supplemental Figure 1). Although Erfe mRNA levels were increased at all ages in the marrow and the spleen of Th3/+ mice compared with WT mice, the induction is smaller when the erythroid expansion is taken into account. In contrast to the transcriptional activation observed in the marrow, the increase in Erfe mRNA expression observed in the spleen of Th3/+ mice can therefore be attributed mostly to the expansion of the erythroid compartment.
Time course of Erfe and Gypa mRNA expression in the marrow and the spleen of WT and Th3/+ mice.Erfe (A) and glycophorin A (Gypa) (B) mRNA expression in the marrow and the spleen was compared between WT and Th3/+ mice at 3, 6, and 12 weeks of age. Erfe mRNA level was significantly elevated at all ages in Th3/+ mice compared with WT mice, and its expression was already maximal in the marrow and spleen of 3-week-old animals. Gypa expression was unchanged in the marrow but significantly elevated in the spleen of Th3/+ mice compared with WT mice. Data shown are means ± SEM and were compared for each point between WT and Th3/+ mice (n = 9-24 per group) by 2-tailed Student t-test. ***P < .001, *P < .05.
Time course of Erfe and Gypa mRNA expression in the marrow and the spleen of WT and Th3/+ mice.Erfe (A) and glycophorin A (Gypa) (B) mRNA expression in the marrow and the spleen was compared between WT and Th3/+ mice at 3, 6, and 12 weeks of age. Erfe mRNA level was significantly elevated at all ages in Th3/+ mice compared with WT mice, and its expression was already maximal in the marrow and spleen of 3-week-old animals. Gypa expression was unchanged in the marrow but significantly elevated in the spleen of Th3/+ mice compared with WT mice. Data shown are means ± SEM and were compared for each point between WT and Th3/+ mice (n = 9-24 per group) by 2-tailed Student t-test. ***P < .001, *P < .05.
We also examined the expression of growth differentiation factor 15 (GDF15) and twisted-gastrulation 1 (TWSG1), bone morphogenetic protein family members that have been proposed as pathological suppressors of hepcidin in thalassemia.24,25 Gdf15 expression was not increased in the marrow and was only mildly increased (less than 2-fold) in the spleen of 6-week-old Th3/+ mice compared with WT mice (supplemental Figure 2A-B). Similarly, Twsg1 expression was slightly decreased in the marrow and unchanged in the spleen of Th3/+ mice compared with WT mice (supplemental Figure 2C-D), suggesting that neither GDF15 nor TWSG1 are likely to play a role in hepcidin regulation in Th3/+ mice.
Polychromatic erythroblasts are the main source of ERFE.
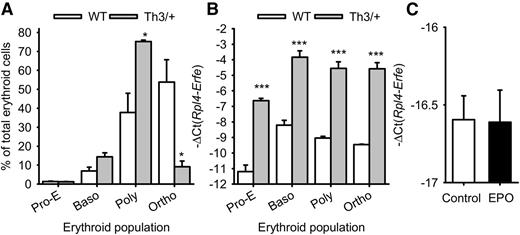
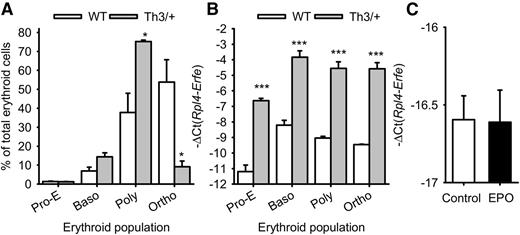
To determine the specific erythroid cell type producing ERFE in β-thalassemia, we sorted the erythroblast populations in the marrow of Th3/+ mice and WT mice. Erythropoiesis in β-thalassemia is characterized by an accumulation of polychromatic erythroblasts that do not mature to the orthochromatic stage (Figure 2A). We found that Erfe expression is increased more than 16-fold in proerythroblasts, basophilic, polychromatic, and orthochromatic erythroblasts isolated from Th3/+ mice compared with those of WT mice, but given their abundance, polychromatic erythroblasts are the predominant source of ERFE in β-thalassemia (Figure 2B). Macrophages do not produce ERFE: Erfe expression was at the detection limit in mouse marrow-derived macrophages, even after 15 hours of treatment with EPO (Figure 2C).
Erfe mRNA expression in erythroid cell populations. Erythroid cells from proerythroblasts (Pro-E) through basophilic (Baso) and polychromatic (Poly) to orthochromatic (Ortho) erythroblasts were isolated from the marrow of 3 WT and 3 Th3/+ mice at 12 weeks of age. (A) The percentage of each cell type (means ± SEM) in the total (Ter119+) erythroid cell population was compared between WT and Th3/+ mice by 2-tailed Student t-test. (B) Erfe mRNA expression (means ± SEM) was compared for each cell type between WT and Th3/+ mice by 2-tailed Student t-test. Erfe mRNA expression was greatly increased in all erythroid cell types. (C) Treatment of WT marrow-derived macrophages with EPO did not influence Erfe mRNA expression. Means ± SEM of 3 independent experiments were compared between controls and EPO-treated cells by 2-tailed Student t-test. ***P < .001, *P < .05.
Erfe mRNA expression in erythroid cell populations. Erythroid cells from proerythroblasts (Pro-E) through basophilic (Baso) and polychromatic (Poly) to orthochromatic (Ortho) erythroblasts were isolated from the marrow of 3 WT and 3 Th3/+ mice at 12 weeks of age. (A) The percentage of each cell type (means ± SEM) in the total (Ter119+) erythroid cell population was compared between WT and Th3/+ mice by 2-tailed Student t-test. (B) Erfe mRNA expression (means ± SEM) was compared for each cell type between WT and Th3/+ mice by 2-tailed Student t-test. Erfe mRNA expression was greatly increased in all erythroid cell types. (C) Treatment of WT marrow-derived macrophages with EPO did not influence Erfe mRNA expression. Means ± SEM of 3 independent experiments were compared between controls and EPO-treated cells by 2-tailed Student t-test. ***P < .001, *P < .05.
Erythrocyte transfusion decreases Erfe mRNA expression.
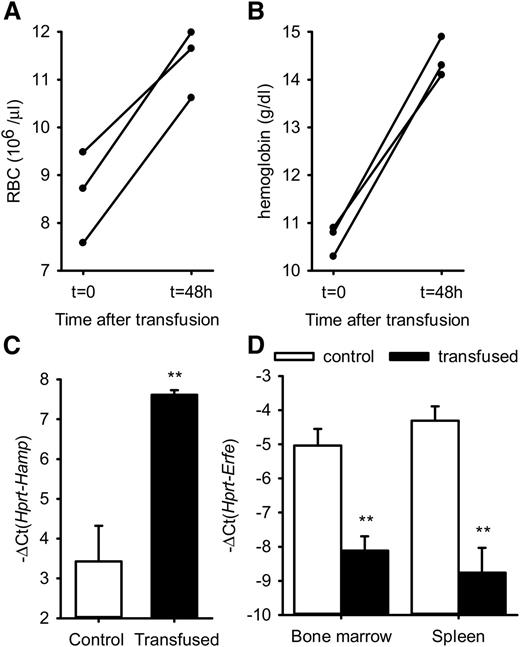
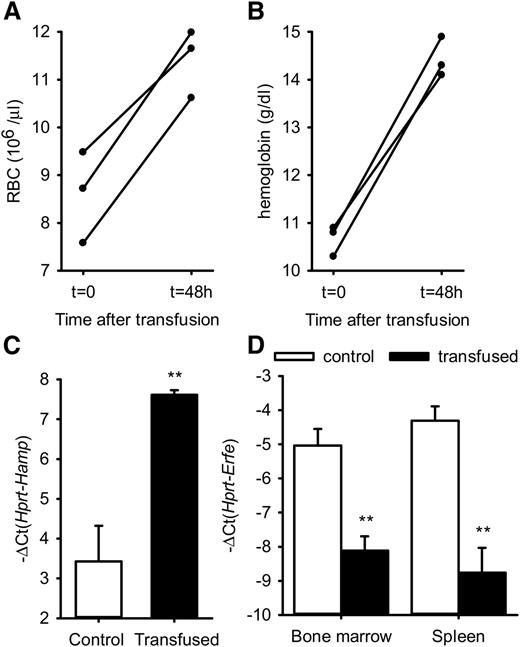
To determine whether ERFE overproduction in β-thalassemia is stimulated by anemia, we tested whether erythrocyte transfusion influences Erfe mRNA expression in thalassemic mice, but not in WT mice in which Erfe levels are already low. Six-week-old thalassemic mice were injected with 400 µL packed red cells or with an equivalent volume of saline and analyzed after 48 hours. Red blood cells (RBC) and hemoglobin were increased to normal ranges in Th3/+ mice 48 hours after transfusion, showing that blood transfusion effectively relieved the anemia in thalassemic mice (Figure 3A-B). We found that hepatic hepcidin expression was increased in transfused mice compared with control mice (Figure 3C). Consistent with the resolution of the anemia, Erfe mRNA expression was reduced to baseline levels in the bone marrow and the spleen of transfused Th3/+ mice compared with control mice (Figure 3D).
RBC, hemoglobin, Hamp mRNA, and Erfe mRNA levels after transfusion in thalassemic mice. RBC (A) and hemoglobin (B) were significantly increased in Th3/+ mice 48 hours after transfusion of 400 µL packed red cells. Hamp mRNA expression was increased in the liver, whereas Erfe mRNA levels were decreased and returned to baseline in the bone marrow and spleen of transfused Th3/+ mice compared with saline-treated control mice. (C-D) Data shown are means ± SEM and were compared between control and transfused Th3/+ mice (n = 3 per group) by 2-tailed Student t-test. **P < .01.
RBC, hemoglobin, Hamp mRNA, and Erfe mRNA levels after transfusion in thalassemic mice. RBC (A) and hemoglobin (B) were significantly increased in Th3/+ mice 48 hours after transfusion of 400 µL packed red cells. Hamp mRNA expression was increased in the liver, whereas Erfe mRNA levels were decreased and returned to baseline in the bone marrow and spleen of transfused Th3/+ mice compared with saline-treated control mice. (C-D) Data shown are means ± SEM and were compared between control and transfused Th3/+ mice (n = 3 per group) by 2-tailed Student t-test. **P < .01.
Circulating levels of ERFE are greatly increased in thalassemia.
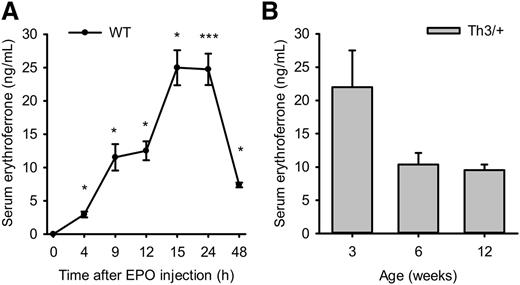
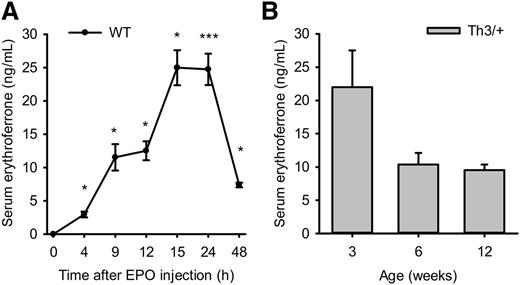
Erfe mRNA expression is greatly stimulated by EPO, but the corresponding plasma ERFE protein levels have not been reported. We measured ERFE concentration in the plasma of EPO-treated WT mice and of untreated littermate WT and Th3/+ mice at 3, 6, and 12 weeks of age. Although ERFE levels were below the lower limit of detection at baseline (less than 100 pg/mL), ERFE concentration increased to 25 ng/mL 15 to 24 hours after EPO injection and progressively decreased between 24 and 48 hours (Figure 4A). Similarly, ERFE was not detectable in 3-, 6-, and 12-week-old WT mice, but Th3/+ mice exhibited massively increased circulating concentration of ERFE (25 ng/mL at 3 weeks of age and 10 ng/mL at 6 and 12 weeks of age) (Figure 4B). Because ERFE has been previously described as a regulator whole-body fatty acid metabolism,26 we assayed ERFE levels in the plasma of WT and Apoe-deficient mice fed 2 months with either a low-fat diet or a Western diet23 (male mice, n = 5 for each of the 4 groups). Interestingly, ERFE was not detectable in any of these groups, suggesting that the production of ERFE during the regulation of energy metabolism may not be stimulated or is negligible compared with its production by erythroid cells during increased erythropoietic activity.
Measurement of plasma ERFE after EPO injection and in thalassemic mice. ERFE concentration was measured in plasma samples of EPO-treated WT mice (A) and littermate WT and Th3/+ mice at 3, 6, and 12 weeks of age (B). (A) ERFE levels in WT mice gradually and significantly increased between 4 and 15 hours after administration of EPO (200 U), reached maximum at 15 and 24 hours, and decreased again by 48 hours. Plasma ERFE concentration was elevated into a similar range in Th3/+ mice (B) at 3, 6, and 12 weeks of age compared with WT mice (P < .001), in which ERFE levels were lower than the limit of detection (100 pg/mL). Data shown are means ± SEM and were compared for each point to values for control mice at t = 0 (n = 4 mice per group and condition) in EPO-treated mice and for each age group between WT and Th3/+ mice (n = 6 to 13 per group) by 2-tailed Student t-test. ***P < .001, *P < .05.
Measurement of plasma ERFE after EPO injection and in thalassemic mice. ERFE concentration was measured in plasma samples of EPO-treated WT mice (A) and littermate WT and Th3/+ mice at 3, 6, and 12 weeks of age (B). (A) ERFE levels in WT mice gradually and significantly increased between 4 and 15 hours after administration of EPO (200 U), reached maximum at 15 and 24 hours, and decreased again by 48 hours. Plasma ERFE concentration was elevated into a similar range in Th3/+ mice (B) at 3, 6, and 12 weeks of age compared with WT mice (P < .001), in which ERFE levels were lower than the limit of detection (100 pg/mL). Data shown are means ± SEM and were compared for each point to values for control mice at t = 0 (n = 4 mice per group and condition) in EPO-treated mice and for each age group between WT and Th3/+ mice (n = 6 to 13 per group) by 2-tailed Student t-test. ***P < .001, *P < .05.
Ablation of Erfe in Th3/+ mice relieves hepcidin suppression.
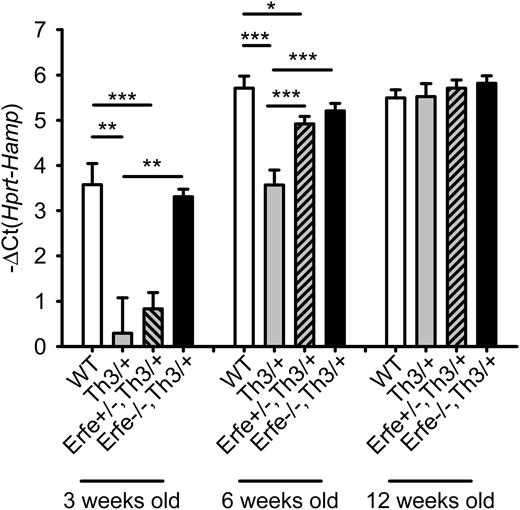
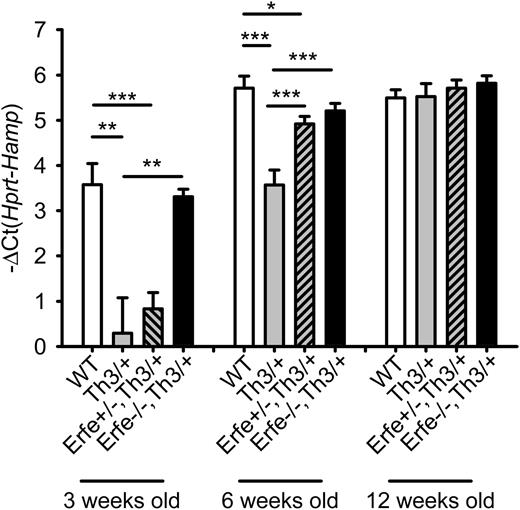
We next investigated the contribution of ERFE-mediated suppression of hepcidin in iron overload and anemia in β-thalassemia in the mouse. In contrast to human patients, hepcidin mRNA expression increases concomitantly with the iron overload, and the deficit in hepcidin mRNA expression is only observed in young Th3/+ mice compared with WT mice. On standard chow (containing about 270 ppm iron; ie, 8 times the daily iron requirement of 35 ppm27 ), by the age of 6 weeks, Th3/+ had similar hepcidin levels and higher liver iron content than WT mice (supplemental Figure 3). To allow the analysis of iron loading in adult mice, the mice were maintained on an iron-sufficient diet containing 50 ppm Fe. We compared littermates WT, Th3/+, and Th3/+ with homozygous or heterozygous ablation of Erfe at 3, 6, and 12 weeks of age (Figure 5). At 3 and 6 weeks of age, hepcidin expression was reduced 8- and 4-fold in the liver of Th3/+ mice (gray bars) compared with WT mice (white bars). Ablation of Erfe in Th3/+ mice (black bars) restored low hepcidin levels to normal. At 6 weeks of age, even heterozygous deletion of Erfe on a thalassemic background (gray dashed bars) was sufficient to significantly increase hepcidin levels compared with Th3/+ mice. By 12 weeks of age, all genotypes reached maximal expression of hepcidin, and no difference was observed between the groups. Our results demonstrate that inactivation of Erfe in a mouse model of β-thalassemia normalizes hepcidin expression to the levels observed in WT animals.
Ablation of ERFE in thalassemic mice restores WT hepcidin levels.Th3/+ mice had significantly lower hepcidin mRNA levels at 3 and 6 weeks of age compared with WT mice. Erfe-deficient mice on a thalassemic background (Erfe−/−, Th3/+) had similar hepcidin to WT mice at 3 and 6 weeks of age. Erfe+/−, Th3/+ mice also showed greatly elevated hepcidin compared with Th3/+ mice at the age of 6 weeks. No difference was observed between the 12-week-old mice. Data shown are means ± SEM and were compared for each age group among WT, Th3/+, Erfe+/−, Th3/+, and Erfe−/−, Th3/+ mice (n = 9 to 36 per group) by 2-tailed Student t-test. ***P < .001, **P < .01, *P < .05.
Ablation of ERFE in thalassemic mice restores WT hepcidin levels.Th3/+ mice had significantly lower hepcidin mRNA levels at 3 and 6 weeks of age compared with WT mice. Erfe-deficient mice on a thalassemic background (Erfe−/−, Th3/+) had similar hepcidin to WT mice at 3 and 6 weeks of age. Erfe+/−, Th3/+ mice also showed greatly elevated hepcidin compared with Th3/+ mice at the age of 6 weeks. No difference was observed between the 12-week-old mice. Data shown are means ± SEM and were compared for each age group among WT, Th3/+, Erfe+/−, Th3/+, and Erfe−/−, Th3/+ mice (n = 9 to 36 per group) by 2-tailed Student t-test. ***P < .001, **P < .01, *P < .05.
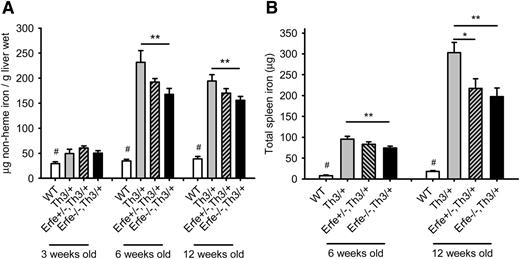
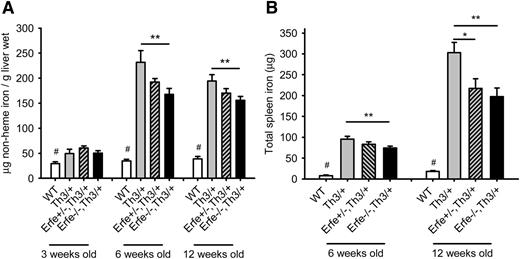
Ablation of Erfe in Th3/+ mice relieves the iron overload.
Consistent with higher hepcidin levels, ablation of Erfe in thalassemic mice led to a significant reduction in liver iron content compared with Th3/+ mice at 6 weeks and at 12 weeks of age (Figure 6A). Similarly, we observed significantly reduced total spleen iron content in Erfe−/−, Th3/+ mice compared with Th3/+ mice at 6 and 12 weeks of age (Figure 6B). At the age of 12 weeks, heterozygous Erfe+/−, Th3/+ mice also exhibited reduced spleen iron compared with Th3/+ mice. No difference in liver iron content was observed at 3 weeks of age between the genotypes, and spleen iron content was undetectable for most of the mice, regardless of their genotype (data not shown). Interestingly, although Erfe ablation decreased liver and spleen iron content of thalassemic mice at 6 weeks of age, they still had higher liver and spleen iron content than WT mice, despite hepcidin levels that were comparable to that of WT mice at both 3 and 6 weeks of age. Because in Erfe-deficient Th3/+ mice these “normal” hepcidin levels were not sufficient to prevent additional iron accumulation, and their slope of hepcidin increase as a function of liver and spleen iron concentration was intermediate between that of Th3/+ and WT mice (supplemental Figure 4), we surmise that in addition to ERFE-mediated hepcidin suppression, additional mechanisms contribute to iron overload: a hepcidin-independent increase of iron absorption and an ERFE-independent mechanism of hepcidin inhibition. As mice progressively increased their hepcidin levels between 3 and 12 weeks of age (Figure 5), iron was no longer accumulating in the liver of thalassemic mice between 6 and 12 weeks of age (Figure 6A), but was retained in the spleen (Figure 6B), so that total spleen iron content was significantly increased in 12-week-old thalassemic mice compared with 6-week-old animals, independent of their Erfe genotype. Erfe−/−, Th3/+ exhibited slightly reduced serum iron concentration compared with WT and Th3/+ mice at 6 weeks of age (supplemental Figure 5).
Ablation of Erfe in thalassemic mice decreases iron overload. Liver (A) and spleen (B) nonheme iron content was measured in WT, Th3/+, Erfe+/−, Th3/+, and Erfe−/−, Th3/+ at 3, 6, and 12 weeks of age. Mice with thalassemic trait mice had higher liver iron content at all ages compared with WT mice. Erfe-deficient mice on a thalassemic background (Erfe−/−, Th3/+) had reduced liver (A) and spleen (B) iron content compared with Th3/+ mice at 6 and 12 weeks of age. No difference in liver and spleen iron content was observed between the genotypes in 3-week-old animals, and the spleen iron content was not detectable in most of the mice at the age of 3 weeks. Data shown are means ± SEM and were compared for each age between Erfe−/−, Th3/+ or Erfe+/−, Th3/+ mice and Th3/+ mice (n = 9-36 per group) by 2-tailed Student t-test. ***P < .001, **P < .01, *P < .05. WT mice had significantly lower liver and spleen iron content compared with all the thalassemic mice (#P < .05 or less). Note that the data in A for 6-week-old mice were previously shown in Figure 7E of Kautz et al.12
Ablation of Erfe in thalassemic mice decreases iron overload. Liver (A) and spleen (B) nonheme iron content was measured in WT, Th3/+, Erfe+/−, Th3/+, and Erfe−/−, Th3/+ at 3, 6, and 12 weeks of age. Mice with thalassemic trait mice had higher liver iron content at all ages compared with WT mice. Erfe-deficient mice on a thalassemic background (Erfe−/−, Th3/+) had reduced liver (A) and spleen (B) iron content compared with Th3/+ mice at 6 and 12 weeks of age. No difference in liver and spleen iron content was observed between the genotypes in 3-week-old animals, and the spleen iron content was not detectable in most of the mice at the age of 3 weeks. Data shown are means ± SEM and were compared for each age between Erfe−/−, Th3/+ or Erfe+/−, Th3/+ mice and Th3/+ mice (n = 9-36 per group) by 2-tailed Student t-test. ***P < .001, **P < .01, *P < .05. WT mice had significantly lower liver and spleen iron content compared with all the thalassemic mice (#P < .05 or less). Note that the data in A for 6-week-old mice were previously shown in Figure 7E of Kautz et al.12
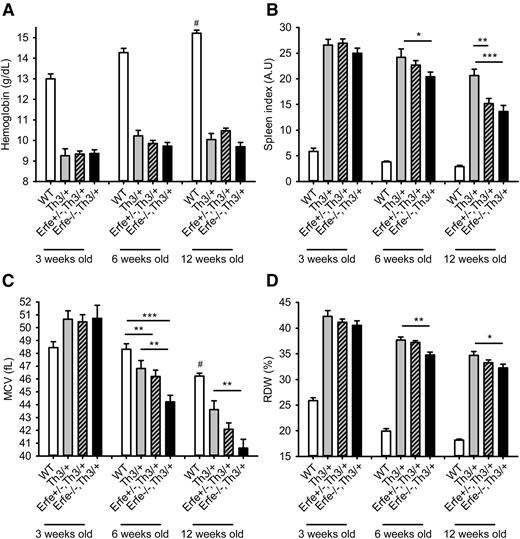
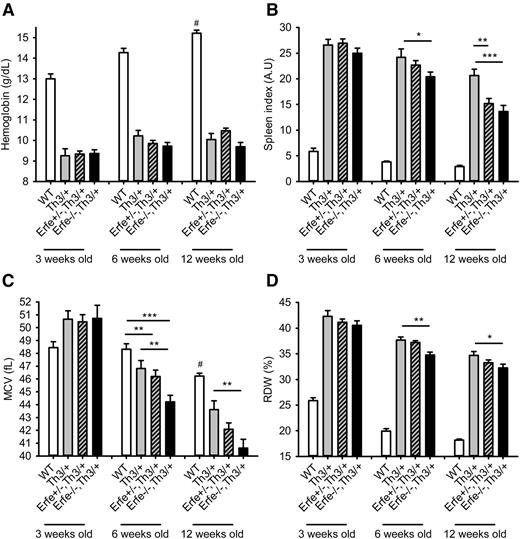
Loss of ERFE does not improve anemia in thalassemic mice.
We next analyzed the hematological parameters in mice at 3 to 12 weeks of age. We observed that compared with Th3/+ mice, thalassemic mice haploinsufficient or deficient for Erfe had similar hemoglobin concentration (Figure 7A), red blood cell count, and hematocrit (supplemental Figure 6), all lower than in WT mice at 3, 6, and 12 weeks of age. Consistent with reduced iron overload, we observed a slight reduction in mean corpuscular volume (MCV), red cell distribution width (RDW) (Figure 7C-D), and MCH (supplemental Figure 7) in Erfe−/−, Th3/+ mice compared with Th3/+ mice at 6 and 12 weeks of age. The most prominent effect of Erfe deletion was on the spleen index, which was reduced in Erfe−/−, Th3/+ mice compared with Th3/+ mice at 6 and 12 weeks of age (20 ± 1 vs 24 ± 1.6 and 13.5 ± 1 vs 21 ± 1, respectively), although it was still higher than in WT mice (Figure 7B). The beneficial effect was more prominent at 12 weeks of age, as even heterozygous Erfe+/−, Th3/+ mice exhibited a decreased spleen index compared with Th3/+ mice (15 ± 1 vs 21 ± 1). However, we did not observe any difference in liver and spleen iron distribution or on peripheral blood smears between Th3/+ and Erfe−/−, Th3/+ mice at 12 weeks of age (supplemental Figure 8). Taken together, these results suggest that disruption of Erfe in thalassemic mice somewhat ameliorates ineffective erythropoiesis but does not improve anemia.
Hemoglobin, spleen index, mean corpuscular volume, and reticulocytes distribution width in WT and thalassemic mice from 3 to 12 weeks of age. Hemoglobin concentration (A) was low in Th3/+ mice and unchanged by manipulation of Erfe. Erfe−/−, Th3/+ had reduced spleen index compared with Th3/+ mice at 6 and 12 weeks of age (B). Erfe+/−, Th3/+ mice also had slightly lower spleen index than Th3/+ mice at the age of 12 weeks. MCV (C) and RDW (D) were significantly reduced in Erfe−/−, Th3/+ mice compared with Th3/+ mice at the age of 6 and 12 weeks. No difference was observed at 3 weeks of age. Data shown are means ± SEM and were compared for each age between Erfe−/−, Th3/+ or Erfe+/−, Th3/+ mice and Th3/+ mice (n = 9-36 per group) by 2-tailed Student t-test. ***P < .001, **P < .01, *P < .05. For comparison, hemoglobin and spleen index were respectively higher and lower in WT mice compared with all the thalassemic mice (#P < .001). MCV was higher at the age of 12 weeks, and RDW was lower at all ages in WT compared with all the thalassemic mice (#P < .001). Note that the data in A and C for 6-week-old WT, Th3/+, and Erfe−/−, Th3/+ mice were previously shown in Figure 15B and 15C, respectively, of Kautz et al.12
Hemoglobin, spleen index, mean corpuscular volume, and reticulocytes distribution width in WT and thalassemic mice from 3 to 12 weeks of age. Hemoglobin concentration (A) was low in Th3/+ mice and unchanged by manipulation of Erfe. Erfe−/−, Th3/+ had reduced spleen index compared with Th3/+ mice at 6 and 12 weeks of age (B). Erfe+/−, Th3/+ mice also had slightly lower spleen index than Th3/+ mice at the age of 12 weeks. MCV (C) and RDW (D) were significantly reduced in Erfe−/−, Th3/+ mice compared with Th3/+ mice at the age of 6 and 12 weeks. No difference was observed at 3 weeks of age. Data shown are means ± SEM and were compared for each age between Erfe−/−, Th3/+ or Erfe+/−, Th3/+ mice and Th3/+ mice (n = 9-36 per group) by 2-tailed Student t-test. ***P < .001, **P < .01, *P < .05. For comparison, hemoglobin and spleen index were respectively higher and lower in WT mice compared with all the thalassemic mice (#P < .001). MCV was higher at the age of 12 weeks, and RDW was lower at all ages in WT compared with all the thalassemic mice (#P < .001). Note that the data in A and C for 6-week-old WT, Th3/+, and Erfe−/−, Th3/+ mice were previously shown in Figure 15B and 15C, respectively, of Kautz et al.12
Discussion
Hepcidin suppression by increased erythropoietic activity underlies the development of iron overload in inherited anemias with ineffective erythropoiesis. This is particularly evident in untransfused patients,2,28 in whom hyperabsorption of dietary iron is the sole source of iron overload. Secondary iron overload, mainly through its cardiac toxicity, is the primary cause of mortality in patients affected with β-thalassemia syndromes. Transfusions partially relieve the ineffective erythropoiesis and raise hepcidin concentrations,29 but worsen the iron overload because of the iron content of transfused blood. Iron chelation therapy, although effective, is not well tolerated by many patients both because of toxic adverse effects (all current chelators) and the burden of parenteral administration (deferoxamine). Alternatives to existing treatments are therefore needed, and hepcidin has become a promising target for novel therapeutic approaches.21 Increasing hepcidin would not reverse established iron overload but could prevent further accumulation of iron and diminish iron-mediated tissue injury by redistributing iron from parenchymal tissues to macrophages30 where iron is less toxic.
The erythroid mediator ERFE,12 produced in response to EPO by erythroblasts in the marrow and the spleen, is a strong suppressor of hepcidin expression. Erfe-deficient mice failed to suppress hepcidin after hemorrhage or EPO administration. Importantly, Erfe mRNA expression was greatly increased in the marrow and the spleen of Th3/+ mice, a mouse model of β-thalassemia intermedia.10 We therefore tested whether increased ERFE levels could be responsible for hepcidin suppression and increased dietary iron absorption in β-thalassemia. Here we showed that Erfe is maximally expressed in Th3/+ mice as early as at 3 weeks of age (Figure 1) and is produced predominantly by polychromatic erythroblasts. In the Th3/+ mouse model of β-thalassemia, these are the most abundant (Figure 2) erythroid precursors, but are largely destined for apoptosis.31 Erythrocyte transfusion relieves the anemia and decreases Erfe mRNA expression in the bone marrow and spleen of Th3/+ mice (Figure 3), and untreated Th3/+ mice have plasma ERFE concentrations similar to those induced by EPO injections into WT mice, supporting the idea that increased plasma ERFE in Th3/+ mice is driven by anemia and the resulting increase in plasma EPO concentrations.
Ablation of Erfe in thalassemic mice restored hepcidin to levels similar to those of WT animals (Figure 5) and led to lower iron accumulation in liver and spleen iron content compared with Th3/+ mice (Figure 6). However, despite hepcidin levels similar to WT mice already at the age of 3 weeks, Erfe-deficient Th3/+ mice still developed progressive iron overload in their livers and spleens. Between 6 and 12 weeks, enough hepatic iron accumulated in all mouse genotypes to raise hepcidin expression to WT levels so that iron no longer increased in the liver but was instead retained in the spleen, especially in Th3/+ mice. Thus, in thalassemic mice the setpoint for hepcidin regulation of iron absorption appears altered so that physiologically normal (WT) levels of hepcidin could not inhibit iron absorption enough to prevent iron overload. Moreover, whereas Erfe-deficient thalassemic mice increased their hepcidin expression in response to iron loading more than Erfe-sufficient thalassemic mice, the increase of hepcidin relative to the liver iron content was still blunted compared with WT mice (supplemental Figure 4). Thus, 2 types of mechanisms may contribute to residual iron overload in the absence of ERFE. First, anemia causes increased duodenal hypoxia, leading to increased HIF2α-enhanced transcription of DMT-1 with a secondary increase in ferroportin mRNA32 and to increased ferroportin on cell membranes of enterocytes despite normal hepcidin expression. In addition, other as-yet-unknown ERFE-independent suppressors of hepcidin could contribute to hepcidin repression in thalassemic mice. Both of these effects can be overcome by pharmacologically increased hepcidin levels, as indicated by the correction of iron overload in Th3/+ mice with ablation of Tmprss6,18 transgenic overexpression of Hamp,8 or treatment with hepcidin agonist minihepcidin.33
Finally, ablation of Erfe in Th3/+ mice did not ameliorate the anemia, as shown by the hemoglobin concentration (Figure 7A), RBC, and hematocrit (supplemental Figure 5), but slightly improved the ineffective erythropoiesis compared with Th3/+ mice, as Erfe−/−, Th3/+ mice exhibited lower spleen index (Figure 7B), MCV, RDW (Figure 7C-D), and MCH (supplemental Figure 7). Amelioration of anemia in this mouse model probably requires higher hepcidin levels and mild iron restriction, which may act to limit α-globin synthesis and reduce the imbalance with β-globin synthesis.8,18
Extrapolation of these findings to humans with β-thalassemia must take into account important differences in their iron metabolism compared with the mouse model of this disease. Unlike adult Th3/+ mice, human adults with β-thalassemia intermedia continue to exhibit marked hepcidin suppression,2-5 suggesting a much greater activity of hepcidin suppressors relative to the hepcidin-stimulatory effect of iron overload. Thus, the mouse model may underestimate the beneficial effect of ERFE antagonism (and hepcidin agonists) in human β-thalassemia. No validated assays to measure plasma ERFE have yet been reported, and the role of ERFE in human pathologies remains to be confirmed. Clinical studies including measurements of plasma ERFE in β-thalassemic patients will be necessary to determine the full potential of these novel therapeutic interventions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Iris Williams for her help in cell sorting. Flow cytometry was performed in the University of California, Los Angeles (UCLA) Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility, supported by National Institutes of Health, National Cancer Institute award P30 CA016042 and National Institutes of Health, National Institute of Allergy and Infectious Diseases award 5P30 AI028697, and by the Jonsson Comprehensive Cancer Center, the UCLA AIDS Institute, the David Geffen School of Medicine at UCLA, the UCLA Chancellor's Office, and the UCLA Vice Chancellor's Office of Research.
This research was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases R01 DK 065029 (to T.G.) and the American Society of Hematology scholar award (to L.K.).
Authorship
Contribution: L.K. designed and performed the experiments, analyzed the data, and wrote the paper. G.J. assisted with experiments. V.G. bred the mice. X.D., J.C., and M.N. developed the ERFE ELISA. E.N. and T.G. supervised the project and wrote the paper.
Conflict-of-interest disclosure: E.N. and T.G. are shareholders and scientific advisors of Intrinsic LifeSciences, Merganser Biotech, and Silarus Therapeutics. T.G. is a consultant to Keryx Biopharmaceuticals. X.D., J.C., and M.N. are shareholders of Silarus Therapeutics.
Correspondence: Léon Kautz, UCLA, Department of Medicine, 10833 LeConte Ave, CHS 37-131, Los Angeles, CA 90095; e-mail: lkautz@mednet.ucla.edu; and Tomas Ganz, UCLA, Department of Medicine, 10833 LeConte Ave, CHS 37-131, Los Angeles, CA 90095; e-mail: tganz@mednet.ucla.edu.