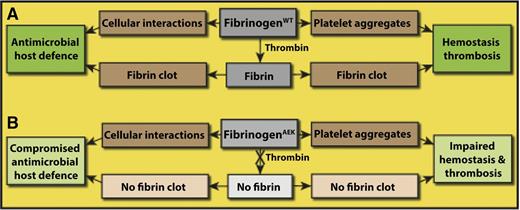
In this issue of Blood, Prasad et al describe a mouse model with a mutation in the Aα chain of fibrinogen such that no fibrin polymer is formed in vivo, allowing for the first time the differentiation of the role of fibrinogen vs fibrin oligomer or polymer in antimicrobial host defense and in hemostasis/thrombosis.1
Role of fibrinogen vs fibrin in antimicrobial host defense and hemostasis/thrombosis. (A) Fibrinogen is involved in hemostasis/thrombosis because it is necessary for platelet aggregation. Similarly, fibrinogen binds specifically to some cells, such as neutrophils and some bacteria, as part of antimicrobial host defense and may have other roles as well. Thrombin cleaves the fibrinopeptides of fibrinogen converting it to fibrin, which aggregates to form a clot. Fibrin clots are a necessary part of hemostasis/thrombosis, but a potential role of fibrin polymer in antimicrobial host defense has not been so clearly demonstrated prior to this paper. (B) In FibAEK mice, fibrinopeptide A cannot be cleaved, so no fibrin clot is formed. These mice show impaired hemostasis and thrombosis and compromised antimicrobial host defense. Hemorrhage is more common, and there is delayed or incomplete vessel occlusion. Survival of FibAEK mice was less than that of wild-type mice but better than fibrinogen knockout mice, and no females survived pregnancy. These mice show severely limited intraperitoneal clearance of S aureus, but increased peritonitis survival compared with afibrinogenemic mice.
Role of fibrinogen vs fibrin in antimicrobial host defense and hemostasis/thrombosis. (A) Fibrinogen is involved in hemostasis/thrombosis because it is necessary for platelet aggregation. Similarly, fibrinogen binds specifically to some cells, such as neutrophils and some bacteria, as part of antimicrobial host defense and may have other roles as well. Thrombin cleaves the fibrinopeptides of fibrinogen converting it to fibrin, which aggregates to form a clot. Fibrin clots are a necessary part of hemostasis/thrombosis, but a potential role of fibrin polymer in antimicrobial host defense has not been so clearly demonstrated prior to this paper. (B) In FibAEK mice, fibrinopeptide A cannot be cleaved, so no fibrin clot is formed. These mice show impaired hemostasis and thrombosis and compromised antimicrobial host defense. Hemorrhage is more common, and there is delayed or incomplete vessel occlusion. Survival of FibAEK mice was less than that of wild-type mice but better than fibrinogen knockout mice, and no females survived pregnancy. These mice show severely limited intraperitoneal clearance of S aureus, but increased peritonitis survival compared with afibrinogenemic mice.
The term fibrin(ogen) has been widely used for many years2 to designate fibrinogen and/or fibrin, because often it is not known if the active molecule in many biological processes is fibrinogen or fibrin or both, because both may be present in vivo. Some examples are antimicrobial host protection, inflammatory reactions, angiogenesis, wound healing, interactions with extracellular matrix components, and fibrin(ogen)olysis.3-6 Surprisingly, although we know that fibrinogen is sufficient for platelet adhesion and aggregation in vitro, we do not even know the relative roles of fibrinogen vs fibrin in platelet aggregation in vivo. It is intuitively clear that insoluble fibrin polymer is made to perform special tasks that are distinct from the roles of the soluble monomeric plasma protein fibrinogen; for example, the mechanical properties of hemostatic clots and thrombi are determined by fibrin, not fibrinogen.7 However, in many other cases, it is virtually impossible to distinguish between the two because there has been no way to fully and selectively control the conversion of fibrinogen to fibrin in vivo.
Now, Prasad et al present a mouse model system that allows the functional distinction between fibrinogen and fibrin to be made (see figure). These studies use a newly developed FibAEK mouse model in which, on a background of the fibrinogen knockout mouse,8 a genetically modified fibrinogen with an Aα chain so that fibrinopeptide A cannot be cleaved by thrombin is knocked in, and hence there is no fibrin formed in vivo or in vitro. There are normal levels of the mutant fibrinogenAEK in the blood, and apparently no other functional properties are affected, so platelet aggregation and probably other fibrinogen functions are maintained. Furthermore, the fibrinogenAEK fibrinopeptide A can be cleaved by enterokinase, and in this case, removal of these peptides is sufficient to allow a clot to form, indicating that there are no structural changes in the mutant molecules that block polymerization. This mouse allows investigation of the roles of fibrinogen vs fibrin polymer, specifically in antimicrobial host defense and hemostasis/thrombosis. Importantly, by comparing the results obtained with the FibAEK mice vs Fib−/− (fibrinogen knockout) mice, it is possible to glean information about the importance of soluble fibrinogen itself, excluding its role as a fibrin precursor.
As an example of the usefulness of this mouse model, the authors studied the effects of this fibrinogen mutation on antimicrobial host defense and found it was compromised, indicating that fibrin and not fibrinogen is primarily involved in these mechanisms. More specifically, FibAEK mice displayed a severe impediment in Staphylococcus aureus clearance following intraperitoneal infection, similar to fibrinogen knockout mice. The mechanism of this effect is not yet defined, but it could be different cell-binding specificity to fibrinogen vs fibrin.9 Conversely, FibAEK mice showed an infection dose-dependent survival advantage over fibrinogen-deficient mice with peritonitis. It should be noted that all of these results are likely to be different for other species of bacteria and modes of infection, which can be variously affected by fibrin. These results have potential importance clinically because of the significance of infection and the design of clinical treatments.
Although the title and much of the text of this paper focus on antimicrobial host defense, these studies also shed some light on the role of fibrin polymerization vs platelet aggregation in hemostasis. Previous studies of fibrinogen knockout mice demonstrated that many of these mice survive to weaning and beyond,8 which might lead casual readers of this paper to conclude that fibrin is not so important. In fact, many of these mice did have severe bleeding, and 100% of females died in pregnancy. These results are consistent with the rare human cases of afibrinogenemia, where bleeding and sometimes thrombosis are serious concerns.10 Of course, the bleeding propensity in hemophilia A and B patients and in factor XIII deficiency also underscores the necessity of fibrin for effective hemostasis. The main finding of the present paper in relation to hemostasis and thrombosis is that FibAEK mice also had a strong propensity for bleeding, which directly indicates that fibrin formation is critically important for hemostasis. The homozygous FibAEK mice had very long tail bleeding times and did not sustain pregnancy due to intrauterine hemorrhage. The superiority of the FibAEK mice over fibrinogen knockout mice in various assays and in survival indicates that fibrinogen-mediated platelet aggregation is certainly important, but fibrin formation is also essential for effective hemostasis. In other words, platelet aggregation cannot rescue the lack of fibrin polymerization in severe bleeding, as observed in liver injury, for example. In addition, the lack of occlusion in the ferric chloride model with FibAEK mice suggests that fibrin polymer is important in thrombosis, which has not been demonstrated so directly heretofore.
This work is a breakthrough in that it opens up the possibility to determine molecular mechanisms in many biological processes and diseases involving fibrinogen and/or fibrin, in that this mouse model can be used for differentiating functions of fibrinogen from those of fibrin. Much work remains to be done with this useful new tool, the FibAEK mouse model.
Conflict-of-interest disclosure: The authors declare no competing financial interests.