Key Points
GVHD elicits profound defects in DCs that prevent the priming of virus-specific T cells.
Transfer of polyclonal T cells from immune donors at transplant provides effective antiviral immunity despite the presence of active GVHD.
Abstract
Viral infection is a common, life-threatening complication after allogeneic bone marrow transplantation (BMT), particularly in the presence of graft-versus-host disease (GVHD). Using cytomegalovirus (CMV) as the prototypic pathogen, we have delineated the mechanisms responsible for the inability to mount protective antiviral responses in this setting. Although CMV infection was self-limiting after syngeneic BMT, in the presence of GVHD after allogeneic BMT, CMV induced a striking cytopathy resulting in universal mortality in conjunction with a fulminant necrotizing hepatitis. Critically, GVHD induced a profound dendritic cell (DC) defect that led to a failure in the generation of CMV-specific CD8+ T-cell responses. This was accompanied by a defect in antiviral CD8+ T cells. In combination, these defects dramatically limited antiviral T-cell responses. The transfer of virus-specific cells circumvented the DC defects and provided protective immunity, despite concurrent GVHD. These data demonstrate the importance of avoiding GVHD when reconstructing antiviral immunity after BMT, and highlight the mechanisms by which the adoptive transfer of virus-specific T cells overcome the endogenous defects in priming invoked by GVHD.
Introduction
Allogeneic hematopoietic stem cell or bone marrow (BM) transplantation (BMT) is a major curative treatment of hematologic malignancies. The therapeutic potential of BMT relates to the immune-mediated graft-versus-tumor (GVT) effect, which is an efficient means to eliminate hematologic malignancies that are refractory to other treatments. One of the major limitations of allogeneic BMT is graft-versus-host disease (GVHD), in which normal recipient tissue is targeted by this immune-mediated process. GVHD is common following allogeneic BMT and accounts for most transplant-related mortality.1 Despite the risk of GVHD, the efficient control of malignant disease makes allogeneic BMT an effective means to eliminate hematologic malignancies at high risk of relapse following standard chemotherapy.
A second important limitation of allogeneic BMT is opportunistic infection. Cytomegalovirus (CMV) represents a predictable and problematic infection after BMT, with ∼70% of patients experiencing viral reactivation.2,3 Historically, CMV was a major cause of posttransplant mortality, with ∼25% of CMV-seropositive recipients developing CMV-related disease within 3 months of transplantation. Although most transplant centers have adopted strict monitoring, preemptive antiviral therapies, or prophylactic strategies to prevent CMV disease, these approaches still have a number of shortcomings (reviewed in Ljungman et al4 ). Therapy requires prolonged administration of toxic antiviral drugs, and life-threatening CMV disease (affecting lung, liver, and/or gastrointestinal tract) still develops in up to 10% of patients. Seropositive recipients who received transplants from seronegative donors (lacking preexisting immunity) are at especially high risk of CMV-related complications.5,6
The control of CMV infection requires the concerted activities of innate and adaptive immune effectors,7 with T-cell–mediated immunity critical to control CMV replication over time and to resolve disease arising from viral reactivation.8 Data in the BMT setting demonstrate that the recovery from CMV disease correlates with reconstitution of the CD8+ T pool,9-14 and provide independent evidence for the crucial role of CD8+ T cells in controlling CMV infection. Indeed, CMV-specific CD8+ T cells can be expanded in vitro and adoptively transferred to treat established CMV disease refractory to antiviral therapy,15-17 or to reduce the need for antiviral pharmacotherapy.11 Although this approach confirms the ability of appropriately activated T cells to limit viral replication, the cellular networks controlling virus after transplantation are poorly understood. In addition, it is clear that in the presence of GVHD, the ability to control CMV is dramatically inhibited,5 but the relevant mechanisms remain unclear. An understanding of the effect of BMT and GVHD on the immunologic control of CMV is critical for the design of effective therapies to prevent complications from this infection.
Materials and methods
Animals
Female BALB/c, DBA/2, congenic CD45.1 C57BL/6J (B6.Ptprca), and CL4 T-cell receptor (TCR)-transgenic mice were from the Animal Resources Centre, Perth. B6.CD11c.DOG (DTR-OVA-enhanced green fluorescent protein [eGFP]) mice were bred at Queensland Institute of Medical Research (QIMR). All mice were housed under specific pathogen-free conditions at the Animal Services Facilities of The University of Western Australia (UWA) and QIMR. All experimentation was performed with the approval of local animal ethics and experimentation committees, according to the National Health and Medical Research Council of Australia guidelines.
BMT
BALB/c and DBA/2 recipients were lethally irradiated with 900 cGy then injected intravenously (IV) with 5 × 106 BALB/c BM cells and 2.5 × 106 BALB/c T cells (prepared from splenocytes using magnetic bead depletion). The development of acute GVHD was monitored by scoring weight loss, reduction in activity, hunching, skin condition, and coat condition (0 = none; 1 = mild; 2 = severe). Mice with combined scores that exceeded 4, prior to or following murine CMV (MCMV) infection, were sacrificed.
Virus infection and quantitation
Mice were inoculated intraperitoneally (IP) with salivary gland propagated stocks of MCMV-K181-Perth, MCMV-K181-Perth–green fluorescent protein (GFP), or MCMV-K181-Perth–hemagglutinin (HA).18 Organs were collected at indicated days postinfection (PI) and processed to determine viral titers by standard plaque assay using M210B4 cells, as described.19
Histology
Organs were collected, preserved in 10% buffered formalin and embedded in paraffin. Tissue sections (5 μm) were stained with hematoxylin and eosin (H&E) or with Croma-10120 (to detect CMV antigen). Slides were examined in a blinded fashion. GVHD was scored using a semiquantitative scoring system.21 Hepatic necrosis is reported as a score that considers severity and extent of necrosis quantified as follows: severity: 1 = several cells (bile duct size area), 2 = small confluent focus (portal vein size area), 3 = zonal necrosis (zone). Extent was measured using a 20× field with an Olympus BX41 microscope in area of maximum involvement: 1 = 1 to 2 foci per field, 2 = 3 to 5 foci per field, 3 = >5 foci per field. Viral inclusions were enumerated. Pictures from tissue sections were taken at room temperature using a Leica DFC295 with application software (version 4.0.0; Leica Microsystems) and a BX41 microscope (for H&E; Olympus) or a DP70 camera with DP71 BW software (version 03.01; Olympus) mounted on a BX60 microscope (for Croma-101; Olympus ).
Liver function tests
Blood was collected by cardiac puncture at the time of sacrifice and heparinized. Alanine transaminase (ALT), aspartate aminotransaminase (AST), and bilirubin levels in plasma were assayed by standard biochemistry (PathWest).
Flow cytometry
Leukocytes were prepared according to standard protocols. Antibodies used for analysis were from BD Biosciences and Biolegend. Phycoerythrin-conjugated tetramers for H-2Ld-YPHFMPTNL MCMV-IE1 and H-2Db HGIRNASFI MCMV-m45 were from ImmunoID Tetramers. At least 100 000 live events were collected using a 6-color FACSCanto (Becton Dickinson) and analyzed using FlowJo analysis software (TreeStar).
Adoptive transfer of T cells
Naive antigen-specific T cells.
Cells were isolated from spleen and lymph nodes of CL4 TCR-transgenic mice (Thy1.1+) and CD8+ T cells prepared by negative bead selection using an EasySep mouse CD8+ T-cell isolation kit (Stemcell Technologies). When required, cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) at a final concentration of 5 μM. CL4 CD8+ T cells (1-2 × 106) were injected IV and identified by Thy1.1 expression.
T cells from naive or immune donors.
Donor T cells were isolated from the spleen of aged-matched naive or MCMV-immune BALB/c mice using magnetic bead depletion. T cells (1-2 × 106) from either naive or immune donors were given to DBA/2 recipients at the time of transplantation.
In vivo DC ablation
B6.Ptprca recipients were lethally irradiated with 1000 cGy then injected IV with 5 × 106 BM and 2 × 106 T cells from B6.CD11c-DOG mice. Diphtheria toxin (DT) (8 ng/g body weight; Sigma Aldrich) or saline was administered IP on days −2, −1, 1, and 3 PI. Dendritic cell (DC) ablation was confirmed by flow cytometric analysis.
T-cell and DC coculture
CD8+ T cells were prepared using an EasySep mouse CD8+ T-cell isolation kit. Conventional DCs (cDCs) were purified from splenocytes using density centrifugation with OptiPrep (Axis-Shield) and positive selection with anti-mouse CD11c microbeads (Miltenyi Biotec). BMDCs were derived by culturing BM cells with Flt3L (250 ng/mL) for 11 days followed by overnight lipopolysaccharide (LPS) stimulation (1 μg/mL). CD8+ T cells (2 × 105) were cultured with 2 × 105 DCs in 200 µL of RPMI–10% fetal calf serum in 96-well round-bottom trays with 5 µg/mL IE1 peptide and 10 ng/mL recombinant human interleukin-2 (hu-IL-2; Cell Sciences).
Statistical analyses
The Student unpaired t test, Mann-Whitney test, or 1-way analysis of variance were performed using InStat Prism software (GraphPad Software).
Results
Mice with acute GVHD are highly susceptible to MCMV cytopathy
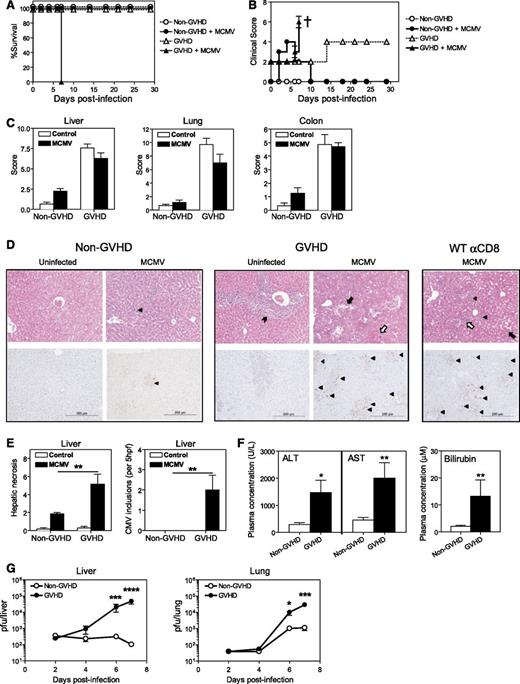
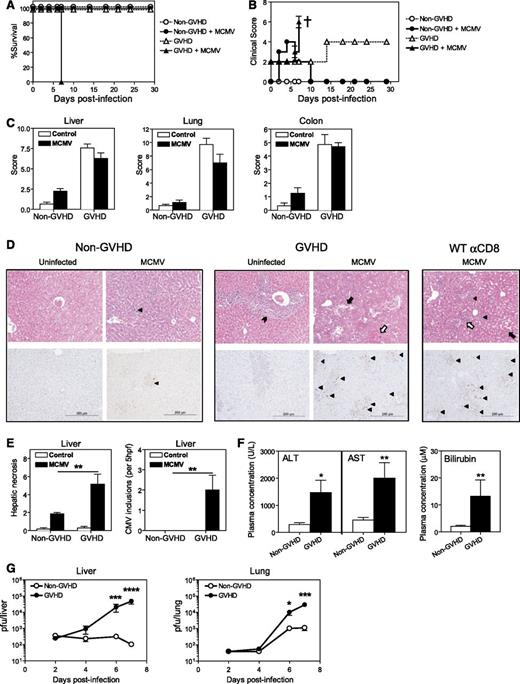
GVHD directed against multiple minor histocompatibility antigens was generated by transplanting lethally irradiated DBA/2 (H-2d) recipients with BM and T cells from BALB/c (H-2d) donors. BALB/c recipients were transplanted with BALB/c grafts to generate syngeneic non-GVHD controls. BMT recipients were infected with MCMV 4 weeks after transplantation. Mice with GVHD became moribund and required sacrifice within 7 days of infection (Figure 1A). Infected mice with GVHD developed high clinical scores and exhibited severe weight loss and increased hunching within 4 to 5 days of infection, followed by a marked decrease in activity by day 6 to 7 PI (Figure 1B). By contrast, uninfected GVHD mice exhibited only mild elevations in clinical score. Non-GVHD mice had minimally elevated clinical scores after MCMV infection, due to the transient weight loss and reduced activity typical of acute CMV viremia.
MCMV infection is lethal in acute GVHD. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. Mice were infected with 5 × 103 plaque-forming units (pfu) MCMV 4 weeks after transplantation. The impact of MCMV infection on (A) survival and (B) clinical scores was assessed. † indicates mice were sacrificed. (C-D) Organs were collected on day 6 PI to (C) grade GVHD pathology, as described.21 (D) H&E (top panels) and immunohistochemical staining for CMV IE1 antigen (bottom panels). These parameters were used to assess CMV-related pathology and CMV viremia. Cytomegalic cells (top panel) or CMV IE1+ cells (brown staining cells in bottom panels) are marked by a filled triangle. Portal tract expansion by an inflammatory cell infiltrate is marked by a filled arrowhead. Areas of hepatic necrosis (filled arrow) and hemorrhagic necrosis (open arrow) are shown. (E) Hepatic necrosis scores (see “Materials and Methods”) and numbers of CMV inclusions are shown. (F) Liver function was assessed at day 6 PI by measuring plasma ALT, AST, and bilirubin levels. (G) Viral loads in the liver and lungs were measured over the course of infection by plaque assay. (A-B) n = 4 to 5 mice per group; (C-E) n = 5 to 8 mice per group; (F) n = 9 to 15 mice per group; (G) n = 5 to 10 mice per group. Data in C-G are representative of, or pooled from, at least 2 independent experiments. *P = .01-.05; **P = .001-.01; ***P = .0001-.001; ****P < .0001.
MCMV infection is lethal in acute GVHD. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. Mice were infected with 5 × 103 plaque-forming units (pfu) MCMV 4 weeks after transplantation. The impact of MCMV infection on (A) survival and (B) clinical scores was assessed. † indicates mice were sacrificed. (C-D) Organs were collected on day 6 PI to (C) grade GVHD pathology, as described.21 (D) H&E (top panels) and immunohistochemical staining for CMV IE1 antigen (bottom panels). These parameters were used to assess CMV-related pathology and CMV viremia. Cytomegalic cells (top panel) or CMV IE1+ cells (brown staining cells in bottom panels) are marked by a filled triangle. Portal tract expansion by an inflammatory cell infiltrate is marked by a filled arrowhead. Areas of hepatic necrosis (filled arrow) and hemorrhagic necrosis (open arrow) are shown. (E) Hepatic necrosis scores (see “Materials and Methods”) and numbers of CMV inclusions are shown. (F) Liver function was assessed at day 6 PI by measuring plasma ALT, AST, and bilirubin levels. (G) Viral loads in the liver and lungs were measured over the course of infection by plaque assay. (A-B) n = 4 to 5 mice per group; (C-E) n = 5 to 8 mice per group; (F) n = 9 to 15 mice per group; (G) n = 5 to 10 mice per group. Data in C-G are representative of, or pooled from, at least 2 independent experiments. *P = .01-.05; **P = .001-.01; ***P = .0001-.001; ****P < .0001.
To determine the nature of the disease observed in transplanted mice following MCMV infection, the visceral GVHD target organs (liver, lungs, and gastrointestinal tract) were examined by histopathology to grade disease severity in an established, semiquantitative fashion.21 The severity of GVHD pathology was equivalent in MCMV-infected and uninfected mice in all tissues examined, with significant GVHD pathology noted in liver, lungs, and the gastrointestinal tract (Figure 1C). Severe pathology associated with viral infection was noted in the livers of MCMV-infected GVHD mice, but was absent in the livers of infected non-GVHD animals (Figure 1D top panels). This pathology was characterized by extensive diffuse hepatic necrosis with large numbers of viral inclusions, and MCMV antigen as detected by immunohistochemistry (Figure 1D bottom panels, E). Consistent with the histologic picture of necrotizing hepatitis, plasma ALT, AST, and bilirubin levels were significantly increased (Figure 1F). Quantification of replicating virus by plaque assay demonstrated a steady increase in MCMV replication in all organs tested, with very high viremic loads observed in the liver and lungs by day 7 PI in mice with GVHD (Figure 1G). By contrast, a resolving infection with decreasing viral loads was noted in the organs of MCMV-infected non-GVHD mice (Figure 1G). Taken together, these data indicate that mice with GVHD were unable to control MCMV replication and were dying with liver failure induced by viral cytopathy. In support of this concept, naive BALB/c mice depleted of CD8+ T cells prior to MCMV infection also exhibited liver necrosis and overt viral infection (Figure 1D) and succumbed to infection by day 7 (data not shown).
A comparison of MCMV replication in BALB/c and DBA/2 mice excluded the possibility that the overt disease observed in the GVHD animals might correlate with an increased susceptibility of DBA/2 mice to MCMV infection (supplemental Figure 1, see supplemental Data available at the Blood Web site).
The antiviral CD8+ T-cell response is impaired in GVHD
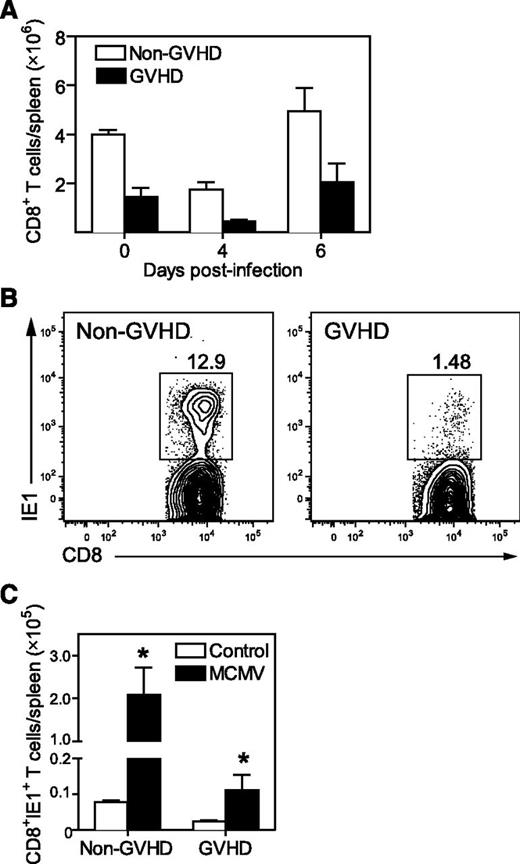
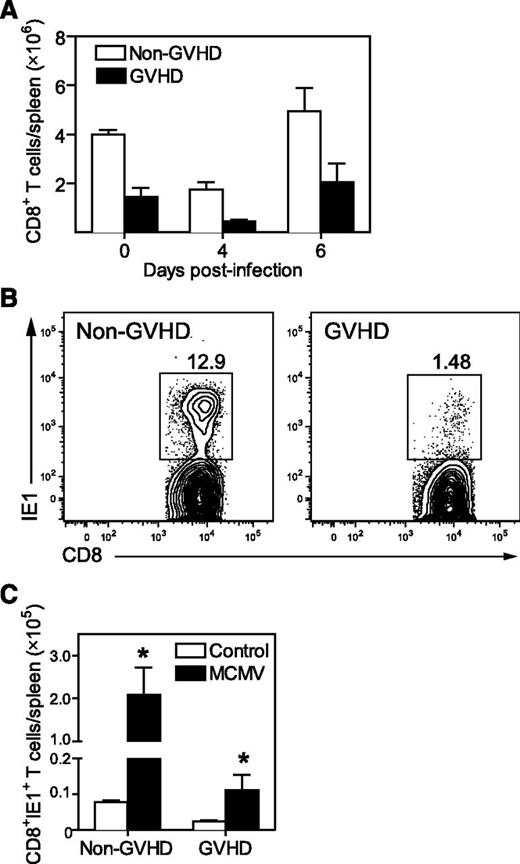
The control of MCMV viremia in BALB/c mice requires an effective antiviral CD8+ T-cell response principally directed to an epitope of the IE1 viral protein.22 IE1-specific CD8+ T cells are readily identified with major histocompatibility complex I (MHC-I; H-2Ld) tetramers.18,23 Both GVHD and non-GVHD mice exhibited some modulation in splenic CD8+ T-cell numbers in response to MCMV infection (Figure 2A), but a much lower frequency of IE1-specific CD8+ T cells was generated in GVHD mice, compared with non-GVHD mice (Figure 2B). As a consequence, ∼20-fold less IE1-specific CD8+ T cells were present in the spleens of GVHD mice on day 7 PI (Figure 2C).
Antiviral CD8+ T-cell immunity is primed weakly during GVHD. BALB/c (non-GVHD) and DBA/2 (GVHD) mice were transplanted with BALB/c BM (5 × 106) and CD3+ T cells (2.5 × 106). Mice were infected with 5 × 103 pfu MCMV 4 weeks after transplantation. (A) Splenic CD8+ T cells were enumerated at the indicated times post-MCMV. Day 0 represent baseline values obtained from uninfected controls. (B) Representative plots showing MCMV-specific CD8+ T cells identified using IE1 tetramers are shown. (C) IE1-specific CD8+ T cells present in the spleen were enumerated on day 7 after MCMV infection, and compared with uninfected controls. (A) n = 3 to 4 mice per group; (B) plot is representative of data from at least 3 independent experiments where n = 5 mice per group; C: n = 4 to 7 mice per group. Data in A and C are representative of at least 2 independent experiments. *P = .01-.05.
Antiviral CD8+ T-cell immunity is primed weakly during GVHD. BALB/c (non-GVHD) and DBA/2 (GVHD) mice were transplanted with BALB/c BM (5 × 106) and CD3+ T cells (2.5 × 106). Mice were infected with 5 × 103 pfu MCMV 4 weeks after transplantation. (A) Splenic CD8+ T cells were enumerated at the indicated times post-MCMV. Day 0 represent baseline values obtained from uninfected controls. (B) Representative plots showing MCMV-specific CD8+ T cells identified using IE1 tetramers are shown. (C) IE1-specific CD8+ T cells present in the spleen were enumerated on day 7 after MCMV infection, and compared with uninfected controls. (A) n = 3 to 4 mice per group; (B) plot is representative of data from at least 3 independent experiments where n = 5 mice per group; C: n = 4 to 7 mice per group. Data in A and C are representative of at least 2 independent experiments. *P = .01-.05.
MCMV infection of DCs is impaired during GVHD
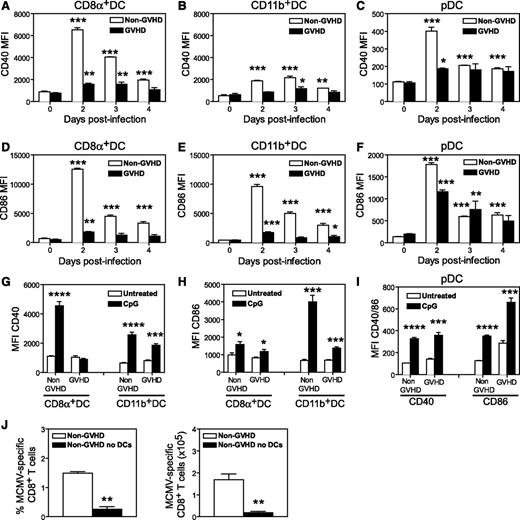
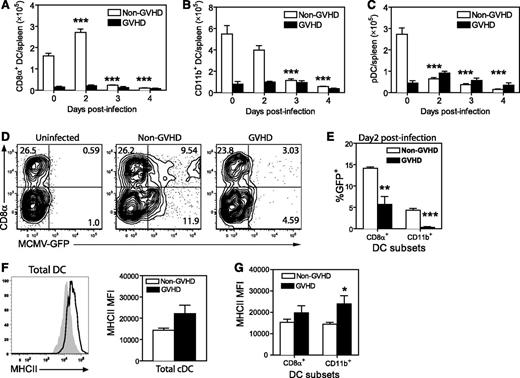
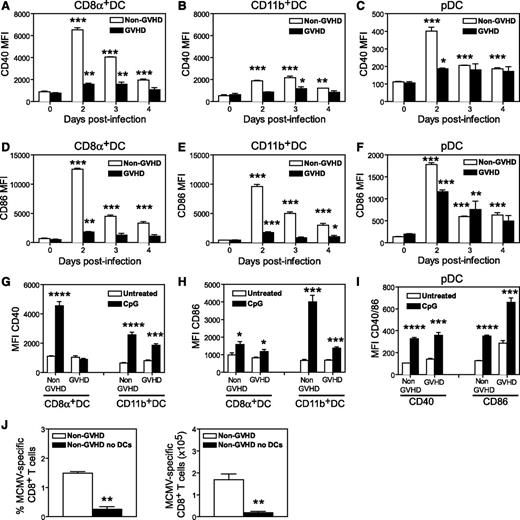
DCs are essential to generate effector T cells during CMV,18,19 especially during a primary response. The reduced number of IE1+ CD8+ T cells generated in MCMV-infected GVHD mice might result from impaired priming. Therefore, we examined the number and activation status of splenic DCs after MCMV infection. Prior to MCMV infection (day 0), GVHD mice exhibited a marked deficit in CD8+ DCs (Figure 3A), CD11b+ DCs (Figure 3B) and plasmacytoid DCs (pDCs) (Figure 3C) compared with non-GVHD mice. MCMV infection induced a loss of all 3 DC populations in non-GVHD mice between day 2 and 3 PI, as previously reported.18,24 In GVHD mice, DC numbers remained very low after infection.
DC numbers are diminished in acute GVHD and DCs are resistant to MCMV infection. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. Four weeks after transplantation, mice were infected with 5 × 103 pfu MCMV. Numbers of (A) CD8α+ DCs, (B) CD11b+ DCs, and (C) pDCs were determined for the spleen at the indicated times after MCMV infection. (D-E) Non-GVHD and GVHD mice were infected with 1 × 104 pfu MCMV-expressing GFP. Spleens were collected on day 2 PI and frequency of GFP+ DCs was determined by flow cytometry. (D) Representative plots are shown for DCs from an uninfected control and 1 mouse from each group of infected mice. (E) The frequencies of CD8α+ DCs and CD11b+ DCs that were GFP+ are shown. (F-G) The expression of MHC-II was determined for splenic cDCs, CD8α+ DCs, and CD11b+ DCs prior to infection. (F) Representative histograms for non-GVHD (filled histogram) vs GVHD mice (bold line), as well as MHC-II mean fluorescence intensity (MFI) for cDCs from non-GVHD and GVHD mice. (G) MHC-II MFI values for splenic CD8α+ DCs and CD11b+ DCs from non-GVHD and GVHD mice are shown. (A-C) 3 to 5 mice per group; (D-E) 3 to 4 mice per group; (F-G) 5 mice per group. Data in D-G are representative of 2 independent experiments. *P = .01-.05, **P = .001-.01, ***P = .0001-.001.
DC numbers are diminished in acute GVHD and DCs are resistant to MCMV infection. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. Four weeks after transplantation, mice were infected with 5 × 103 pfu MCMV. Numbers of (A) CD8α+ DCs, (B) CD11b+ DCs, and (C) pDCs were determined for the spleen at the indicated times after MCMV infection. (D-E) Non-GVHD and GVHD mice were infected with 1 × 104 pfu MCMV-expressing GFP. Spleens were collected on day 2 PI and frequency of GFP+ DCs was determined by flow cytometry. (D) Representative plots are shown for DCs from an uninfected control and 1 mouse from each group of infected mice. (E) The frequencies of CD8α+ DCs and CD11b+ DCs that were GFP+ are shown. (F-G) The expression of MHC-II was determined for splenic cDCs, CD8α+ DCs, and CD11b+ DCs prior to infection. (F) Representative histograms for non-GVHD (filled histogram) vs GVHD mice (bold line), as well as MHC-II mean fluorescence intensity (MFI) for cDCs from non-GVHD and GVHD mice. (G) MHC-II MFI values for splenic CD8α+ DCs and CD11b+ DCs from non-GVHD and GVHD mice are shown. (A-C) 3 to 5 mice per group; (D-E) 3 to 4 mice per group; (F-G) 5 mice per group. Data in D-G are representative of 2 independent experiments. *P = .01-.05, **P = .001-.01, ***P = .0001-.001.
Direct presentation of viral antigens by infected DCs is critical for the generation of a protective IE1-specific CD8+ response in BALB/c mice.18,19 We examined the infection of DCs using a recombinant MCMV-expressing GFP.25 Spleens were collected from non-GVHD and GVHD mice 2 days PI, and GFP expression in DCs was measured by flow cytometry (Figure 3D). GFP was readily detected in both CD8+ and CD11b+ DCs from non-GVHD mice, whereas very few DCs were GFP+ in mice with GVHD (Figure 3E), and the level of infection (determined by GFP fluorescence intensity) was also reduced (Figure 3D).
We have previously shown that LPS-activated DCs are more resistant to MCMV infection than immature DCs.24 Similarly, activation of DCs in vivo impairs presentation.26 The onset of GVHD is associated with activation/maturation of splenic DCs, producing mild upregulation of MHC-II and some costimulatory molecules at day 10 posttransplantation.27 MHC-II expression on splenic DCs from GVHD mice prior to infection (∼day 28 posttransplantation) was increased compared with non-GVHD animals (Figure 3F). MHC-II expression was increased in both CD8+ and CD11b+ DC subsets, but was most prominent in the CD11b+ DC population (Figure 3G). Together, these data suggest that splenic DCs were resistant to MCMV infection in mice with GVHD, probably due to their heightened activation/maturation.
MCMV infection fails to activate DCs during GVHD
Next, we examined the expression of costimulatory molecules on splenic DCs in response to MCMV infection. In non-GVHD mice, MCMV induced marked upregulation of CD40 and CD86 on CD8+ DCs, however, a much weaker response was noted in infected GVHD mice (Figure 4A and D, respectively). A similar response was observed in CD11b+ DCs (Figure 4B,E) and pDCs (Figure 4C,F), with the upregulation of CD40 and CD86 consistently lower in GVHD mice. These observations prompted us to test the response of DCs to CpG oligodeoxynucleotide, a Toll-like receptor 9 (TLR9) ligand known to activate DCs. In mice with GVHD, CpG treatment failed to induce upregulation of CD40 on CD8+ DCs (Figure 4G), and upregulation of CD86 on CD11b+ DCs was weaker (Figure 4H) compared with that observed in CpG-treated, non-GVHD mice. CpG administration induced equivalent upregulation of CD40 and CD86 in pDCs from mice with or without GVHD (Figure 4I). Thus, GVHD produces a defect in CD8+ and CD11b+ DC activation in response to MCMV and CpG signaling.
DCs are not efficiently activated by MCMV or TLR ligands during acute GVHD. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. Four weeks after transplantation, mice were either infected with 5 × 103 pfu MCMV (A-F) or administered 100 mM CpG IP (G-I) and the mean fluorescence of CD40 and CD86 expression on DC subsets was determined. (A,D) Data for splenic CD8α+ DC; (B,E) Data for splenic CD11b+ DCs; (C,F) data for splenic pDCs. Spleen cells were collected 48 hours after CpG administration and the mean fluorescence of CD40 (G) and CD86 (H) expression was determined for CD8α+ and CD11b+ DCs. (I) The mean fluorescence of CD40 and CD86 expression on pDCs. Changes between the untreated and CpG-treated groups were tested for significance. (J) B6.Ptprca mice were transplanted with B6.CD11c.DOG BM cells (5 × 106) and T cells (2 × 106), and 4 weeks later they were infected with 5 × 103 pfu MCMV. CD11c.DOG chimeric mice were treated with saline (non-GVHD) or diphtheria toxin on days −2, −1, 1, and 3 to ablate DCs (non-GVHD no DCs). The frequency and number of MCMV-specific CD8+ T cells detected with an m45-specific tetramer are shown. (A-F) 3 to 5 mice per group; (G-I) 4 mice per group; (J) 6 mice per group. *P = .01-.05; **P = .001-.01; ***P = .0001-.001; ****P < .0001.
DCs are not efficiently activated by MCMV or TLR ligands during acute GVHD. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. Four weeks after transplantation, mice were either infected with 5 × 103 pfu MCMV (A-F) or administered 100 mM CpG IP (G-I) and the mean fluorescence of CD40 and CD86 expression on DC subsets was determined. (A,D) Data for splenic CD8α+ DC; (B,E) Data for splenic CD11b+ DCs; (C,F) data for splenic pDCs. Spleen cells were collected 48 hours after CpG administration and the mean fluorescence of CD40 (G) and CD86 (H) expression was determined for CD8α+ and CD11b+ DCs. (I) The mean fluorescence of CD40 and CD86 expression on pDCs. Changes between the untreated and CpG-treated groups were tested for significance. (J) B6.Ptprca mice were transplanted with B6.CD11c.DOG BM cells (5 × 106) and T cells (2 × 106), and 4 weeks later they were infected with 5 × 103 pfu MCMV. CD11c.DOG chimeric mice were treated with saline (non-GVHD) or diphtheria toxin on days −2, −1, 1, and 3 to ablate DCs (non-GVHD no DCs). The frequency and number of MCMV-specific CD8+ T cells detected with an m45-specific tetramer are shown. (A-F) 3 to 5 mice per group; (G-I) 4 mice per group; (J) 6 mice per group. *P = .01-.05; **P = .001-.01; ***P = .0001-.001; ****P < .0001.
Given the multiple DC defects observed during GVHD, we examined how important these antigen-presenting cells (APCs) are for generating antiviral CD8+ T-cell responses by depleting CD11c+ DCs in syngeneic transplants. B6.CD11c.DOG marrow was transplanted into wild-type (WT) B6.Ptprca recipients, mice were then treated with DT prior to MCMV infection, and antiviral CD8+ T-cell responses measured after infection. CMV-specific CD8+ T-cell responses could not be generated in the absence of CD11c+ DCs (Figure 4J), conclusively establishing their essential role in priming antiviral immunity after BMT.
PD1 expression is increased during GVHD and MCMV infection
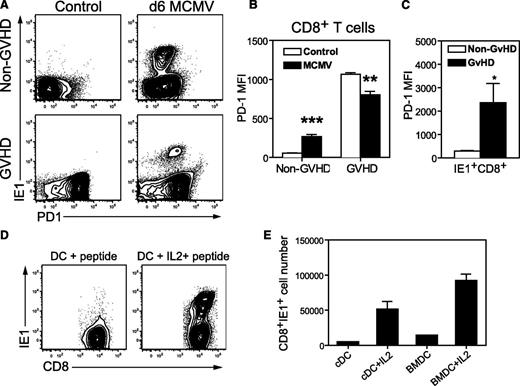
Next, we investigated the expression of PD1 on CD8+ T cells from GVHD and non-GVHD mice, before and after MCMV infection. As expected, PD1 expression was elevated on the majority of splenic CD8+ T cells from mice with GVHD, compared with the low-level expression observed on CD8+ T cells from non-GVHD mice (Figure 5A). MCMV infection induced moderate levels of PD1 expression on splenic CD8+ T cells of non-GVHD mice (Figure 5A-B), including all IE1-specific CD8+ T cells at day 6 PI (Figure 5A,C). PD1 expression remained high on total CD8+ T cells from GVHD mice after MCMV infection (Figure 5A), despite a small, but significant decrease in mean fluorescence compared with uninfected controls (Figure 5B). Notably, in GVHD mice, PD1 was highly expressed on IE1+ CD8+ T cells, with mean fluorescence fivefold to 10-fold higher than in IE+CD8+ T cells from non-GVHD mice (Figure 5A,C).
Antiviral CD8+ T cells express high levels of PD1 during GVHD, but can be expanded ex vivo. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. Mice were infected with 5 × 103 pfu MCMV 4 weeks after transplantation, and spleen cells were harvested on day 6 after MCMV infection. (A-C) The expression of PD1 was determined for CD8+ T cells in conjunction with IE1-specific tetramer binding, and compared with uninfected control mice. (A) Representative plots depicting the expression of PD1 and IE1-tetramer binding by splenic CD8+ T cells. (B) The mean fluorescence for PD1 expression by CD8+ T cells and (C) IE1-specific CD8+ T cells. (D-E) CD8+ T cells were purified from the spleens of infected GVHD mice and cultured for 5 days with DCs and IE1 peptide, with and without 10 ng/mL IL-2. (D) Representative plots depicting IE1+ CD8+ T cells. (E) The number of IE1-specific CD8+ T cells recovered after coculture with DCs. (A-C) n = 4 mice per group, representative of at least 3 independent experiments; (D-E) data are from 1 experiment with a pool of 6 mice. *P = .01-.05, **P = .001-.01, ***P = .0001-.001. BMDC, DCs prepared in vitro after culturing BM from uninfected BALB/c with Flt3 ligand for 11 days; cDC, conventional DCs enriched from uninfected BALB/c spleens.
Antiviral CD8+ T cells express high levels of PD1 during GVHD, but can be expanded ex vivo. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. Mice were infected with 5 × 103 pfu MCMV 4 weeks after transplantation, and spleen cells were harvested on day 6 after MCMV infection. (A-C) The expression of PD1 was determined for CD8+ T cells in conjunction with IE1-specific tetramer binding, and compared with uninfected control mice. (A) Representative plots depicting the expression of PD1 and IE1-tetramer binding by splenic CD8+ T cells. (B) The mean fluorescence for PD1 expression by CD8+ T cells and (C) IE1-specific CD8+ T cells. (D-E) CD8+ T cells were purified from the spleens of infected GVHD mice and cultured for 5 days with DCs and IE1 peptide, with and without 10 ng/mL IL-2. (D) Representative plots depicting IE1+ CD8+ T cells. (E) The number of IE1-specific CD8+ T cells recovered after coculture with DCs. (A-C) n = 4 mice per group, representative of at least 3 independent experiments; (D-E) data are from 1 experiment with a pool of 6 mice. *P = .01-.05, **P = .001-.01, ***P = .0001-.001. BMDC, DCs prepared in vitro after culturing BM from uninfected BALB/c with Flt3 ligand for 11 days; cDC, conventional DCs enriched from uninfected BALB/c spleens.
High levels of PD1 expression have been associated with T-cell exhaustion in some models of chronic viral infection28,29 ; and in GVHD, PD1-PDL1 interactions dampen alloreactive T-cell responses.30,31 When CD8+ T cells purified from the spleens of GVHD mice on day 6 after MCMV infection were cocultured with DCs and IE1 peptide, expansion of IE1-specific CD8+ T cells was only attained when IL-2 was added to the cultures (Figure 5D-E). The PD1 expression data, together with the requirement for exogenous IL-2 for expansion of IE1-specific CD8+ T cells, suggest that virus-specific CD8+ T cells generated in the setting of GVHD may be exhausted.
The priming of naive T cells is attenuated during GVHD
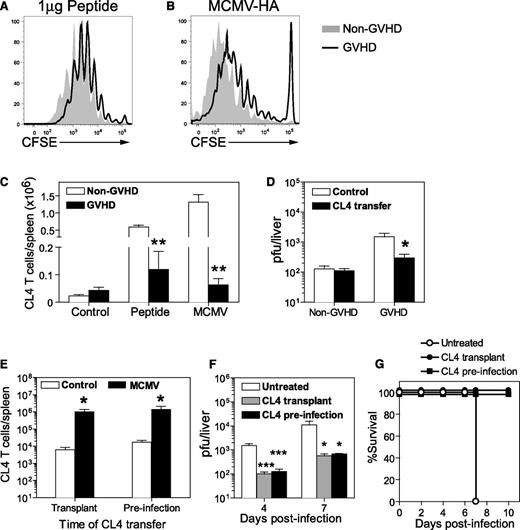
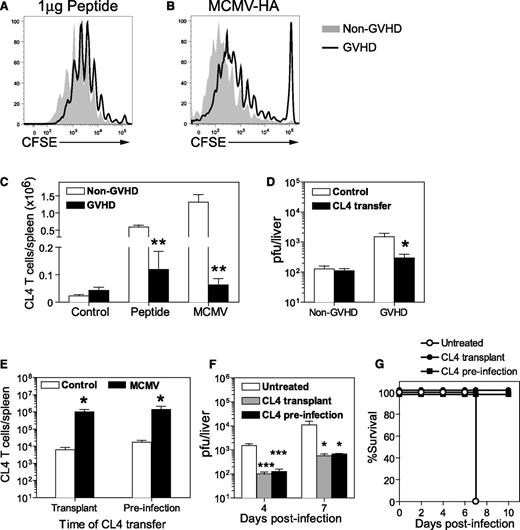
Having identified a defect in DC numbers, as well as DC infection and activation in GVHD mice, we used an adoptive transfer system to assess antigen presentation in this setting. This system uses naive CD8+ T cells from CL4 TCR-transgenic mice expressing a TCR that specifically recognizes an epitope (aa 512-520) from influenza HA presented by H-2Kd. Non-GVHD and GVHD mice received CL4 peptide or were infected with a recombinant MCMV expressing the CL4 epitope (MCMV-HA) 1 day after the adoptive transfer of CL4 T cells. Transferred CD8+ T cells were labeled with CFSE to allow proliferation to be measured. Multiple cell divisions were observed in the spleens of mice that received HA peptide (Figure 6A) or were infected with MCMV-HA (Figure 6B). CL4 cells consistently exhibited greater proliferation in non-GVHD mice, resulting in fivefold to 20-fold greater cell expansion than that observed in GVHD mice (Figure 6C). Despite the weaker expansion, CL4 CD8+ T cells effectively controlled MCMV-HA infection in GVHD mice, with viral loads in liver reduced to barely detectable levels, similar to those observed in non-GVHD mice (Figure 6D).
Naive antigen-specific T cells respond to viral antigen in vivo and protect the host from MCMV infection during GVHD. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. (A-D) CD8+ T cells were collected from CL4 TCR-transgenic mice, labeled with CFSE and adoptively transferred to BALB/c (non-GVHD) or DBA/2 (GVHD) mice 4 weeks after transplantation. The proliferation of CL4 CD8+ T cells was measured 3 days after (A) administration of 1 µg CL4 peptide IV, or (B) 4 days after infection with 5 × 103 pfu MCMV-HA, a recombinant MCMV expressing the CL4 epitope. (C) The number of transferred CL4 CD8+ T cells was determined in the spleen for each group. (D) Both groups of mice were infected with 5 × 103 pfu MCMV-HA and viral loads in the liver at day 6 PI determined by plaque assay. (E-G) DBA/2 mice were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. One group received 1 × 106 CL4 CD8+ T cells at the time of transplant (CL4 transplant), 1 group received 1 × 106 CL4 CD8+ T cells 4 weeks later (CL4 preinfection), and 1 group did not receive CL4 CD8+ T cells. All 3 groups of mice were infected with 1 × 104 pfu (E) or 5 × 103 pfu (F) MCMV-HA 4 weeks after transplantation. (E) CL4 CD8+ T cells in the spleen were enumerated at 7 days PI. (F) Viral loads in the liver, as measured by plaque assay, and (G) survival were compared in mice that received CL4 cells or were left untreated. (A-C) n = 3 to 4 mice per group; (D) n = 5 to 6 mice per group; (E-G) 3 to 5 mice per group. Data in D-G are representative of at least 2 independent experiments. *P = .01-.05; **P = .001-.01; ***P = .0001-.001.
Naive antigen-specific T cells respond to viral antigen in vivo and protect the host from MCMV infection during GVHD. BALB/c (non-GVHD) and DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. (A-D) CD8+ T cells were collected from CL4 TCR-transgenic mice, labeled with CFSE and adoptively transferred to BALB/c (non-GVHD) or DBA/2 (GVHD) mice 4 weeks after transplantation. The proliferation of CL4 CD8+ T cells was measured 3 days after (A) administration of 1 µg CL4 peptide IV, or (B) 4 days after infection with 5 × 103 pfu MCMV-HA, a recombinant MCMV expressing the CL4 epitope. (C) The number of transferred CL4 CD8+ T cells was determined in the spleen for each group. (D) Both groups of mice were infected with 5 × 103 pfu MCMV-HA and viral loads in the liver at day 6 PI determined by plaque assay. (E-G) DBA/2 mice were transplanted with BALB/c BM (5 × 106) and CD3+ T (2.5 × 106) cells. One group received 1 × 106 CL4 CD8+ T cells at the time of transplant (CL4 transplant), 1 group received 1 × 106 CL4 CD8+ T cells 4 weeks later (CL4 preinfection), and 1 group did not receive CL4 CD8+ T cells. All 3 groups of mice were infected with 1 × 104 pfu (E) or 5 × 103 pfu (F) MCMV-HA 4 weeks after transplantation. (E) CL4 CD8+ T cells in the spleen were enumerated at 7 days PI. (F) Viral loads in the liver, as measured by plaque assay, and (G) survival were compared in mice that received CL4 cells or were left untreated. (A-C) n = 3 to 4 mice per group; (D) n = 5 to 6 mice per group; (E-G) 3 to 5 mice per group. Data in D-G are representative of at least 2 independent experiments. *P = .01-.05; **P = .001-.01; ***P = .0001-.001.
Adoptive transfer of antigen-specific T cells confers protection against MCMV infection even in the presence of GVHD
The results presented in the previous sections demonstrated that it is possible to generate protective effector T-cell responses from naive antigen-specific CD8+ T cells transferred 1 day prior to infection, yet a protective T-cell response could not be generated from endogenous donor CD8+ T cells in the presence of GVHD. This difference raised the possibility that transplanted CD8+ T cells may be eliminated or rendered unresponsive by GVHD.32,33 We tested this hypothesis by transferring CL4 cells into GVHD mice at 1 of 2 times: at the time of transplantation (CL4 transplant) or 1 day prior to MCMV-HA infection (CL4 infection). Both groups of mice were infected at the same time (ie, 4 weeks posttransplant).
Expansion of CL4-transgenic CD8+ T cells in the spleen was equivalent in both groups of recipients (Figure 6E), indicating that GVHD did not affect the capacity of CL4 T cells to proliferate in response to MCMV-HA. Significantly reduced viral loads were observed in the liver of both groups of mice that received CL4 CD8+ T cells, compared with untreated GVHD mice (Figure 6F). Importantly, CL4 transfer protected from fulminant necrotizing hepatitis and resulted in survival (Figure 6G). These data clearly establish that adoptive transfer of naive donor-matched antigen-specific T cells can protect the host against MCMV infection, regardless of whether these cells are administered at the time of transplant or after the onset of GVHD.
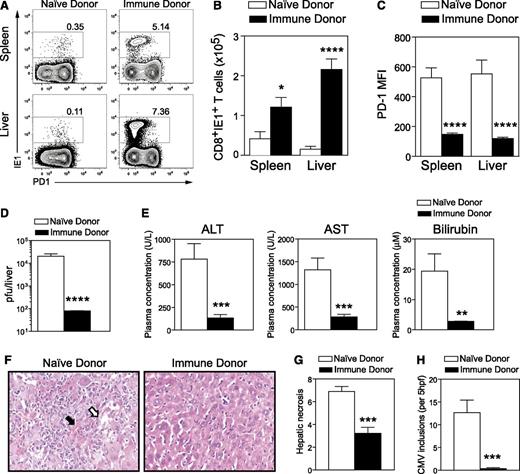
Having shown that naive antigen-specific T cells were capable of responding during GVHD, we next examined whether this holds true for a polyclonal T-cell population. To test this, we transferred T cells from naive donors or polyclonal antigen-experienced T cells from MCMV immune donors into GVHD mice at the time of transplantation and evaluated their capacity to respond to subsequent MCMV infection. Expansion of antiviral IE1+CD8+ T cells was observed in both the spleen and liver (Figure 7A-B) of mice that received T cells from immune donors. Interestingly, expression of PD-1 was significantly lower on antiviral IE1+CD8+ T cells generated in mice that received T cells from immune donors (Figure 7A,C). A significant reduction in viral load was noted in the liver of mice that received T cells from immune donors, compared with mice that received T cells from naive donors (Figure 7D). Importantly, the transfer of T cells from immune donors fully protected GVHD mice from MCMV-induced hepatic disease, as indicated by normal liver biochemistry (Figure 7E), lack of liver pathology (Figure 7F-G), and absence of MCMV inclusions in this organ (Figure 7H).
Antigen-experienced T cells from immune donors respond to MCMV infection during GVHD and confer protection from CMV disease. DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T cells (2.5 × 106) from either naive or MCMV immune donors (latently infected mice). Mice were infected with 5 × 103 pfu MCMV 4 weeks posttransplant and assessed for CD8+ T-cell responses (day 6 PI), viral titers, and liver function (day 6 and 7 PI). (A) Representative plots depicting the expression of PD1 and IE1-tetramer binding by splenic and liver CD8+ T cells from GVHD mice that received polyclonal T cells from either naive or MCMV immune donors. (B) Number of MCMV-specific IE1+CD8+ T cells in spleen and liver, (C) mean fluorescence of PD1 expression on MCMV-specific IE1+CD8+ T cells in spleen and liver, (D) viral loads in the liver (< 102 pfu represents limit of detection), and (E) liver function assessed by measuring plasma concentration of ALT, AST, and bilirubin are shown. Liver disease was further assessed by histologic analysis. (F) H&E staining of liver sections. (G) Hepatic necrosis scores (see “Materials and Methods”) and (H) numbers of CMV inclusions are shown. Data are pooled from 2 independent experiments (11-12 mice per group). *P = .01-.05; **P = .001-.01; ***P = .0001-.001; ****P < .0001.
Antigen-experienced T cells from immune donors respond to MCMV infection during GVHD and confer protection from CMV disease. DBA/2 (GVHD) were transplanted with BALB/c BM (5 × 106) and CD3+ T cells (2.5 × 106) from either naive or MCMV immune donors (latently infected mice). Mice were infected with 5 × 103 pfu MCMV 4 weeks posttransplant and assessed for CD8+ T-cell responses (day 6 PI), viral titers, and liver function (day 6 and 7 PI). (A) Representative plots depicting the expression of PD1 and IE1-tetramer binding by splenic and liver CD8+ T cells from GVHD mice that received polyclonal T cells from either naive or MCMV immune donors. (B) Number of MCMV-specific IE1+CD8+ T cells in spleen and liver, (C) mean fluorescence of PD1 expression on MCMV-specific IE1+CD8+ T cells in spleen and liver, (D) viral loads in the liver (< 102 pfu represents limit of detection), and (E) liver function assessed by measuring plasma concentration of ALT, AST, and bilirubin are shown. Liver disease was further assessed by histologic analysis. (F) H&E staining of liver sections. (G) Hepatic necrosis scores (see “Materials and Methods”) and (H) numbers of CMV inclusions are shown. Data are pooled from 2 independent experiments (11-12 mice per group). *P = .01-.05; **P = .001-.01; ***P = .0001-.001; ****P < .0001.
Thus, the transfer of polyclonal T cells from an immune donor bypasses the defects in DC-mediated priming and protects recipients with active GVHD from MCMV infection.
Discussion
CMV remains a significant clinical problem for recipients of allogeneic BMT despite major advances in diagnosis and disease management.34 Our model of primary MCMV infection during acute GVHD mimics human CMV (HCMV) disease that occurs after BMT when donors are seronegative and no preexisting donor immunity exists. Although there is still some debate about the impact of various donor-recipient serotype subsets,2,35 there is evidence that seropositive recipients who received transplants from seronegative donors are at the highest risk of CMV-related complications.4 Consistent with this, MCMV infection was lethal for mice suffering acute GVHD. In this setting, infection had no effect on the magnitude or progress of GVHD over the viremic period; instead, mice succumbed to a fulminant necrotizing hepatitis directly associated with viral-induced cytopathy. Overt viral-related pathology was not observed in other target organs, namely the lung and gastrointestinal tract. This most likely correlates with the kinetics of viral spread and replication. In this setting, viral replication commences in spleen and liver within 2 days of infection, whereas in the lung CMV replication is not detected until later (day 6). In mice with GVHD, liver disease is so overt by day 6 postinfection that euthanasia is required before overt viral cytopathy can be established in other organs.
An important conclusion of our studies is that GVHD is itself a major risk factor for CMV disease because it severely compromises the induction of an effective adaptive immune response against the virus. In immunocompetent individuals, primary CMV infection is controlled by the combined activities of innate immune effectors, principally natural killer cells, and virus-specific CD8+ T cells.36,37 Although CMV can have cytopathic effects, in the presence of an effective immune response it causes minimal or no pathology.36 In mouse models, CD8+ T cells are critical for protection, and in their absence lethal CMV disease ensues.36 Consistent with this, the liver pathology observed in GVHD closely resembled that seen for mice depleted of CD8+ T cells. Although cross-presentation is generally considered the primary pathway for generating antiviral CD8+ T-cell responses, our studies have demonstrated that protective antiviral T-cell immunity to MCMV depends on direct presentation.19 Furthermore, we have shown that direct presentation depends upon the capacity of MCMV to efficiently infect DCs, mainly the CD11b+ subset,24 with the extent of DC infection being a critical determinant of the magnitude and longevity of antiviral T-cell responses.18 We have previously shown that MCMV infection of DCs is measurable from day 2, increases at day 4, and is sustained until day 6 PI.18 The DC phenotype changes following MCMV infection, with a loss of MHC-II expression being one of the main features.24 Consistent with the latter, the loss of DCs observed in non-GVHD mice from day 3 after MCMV infection is due to the phenotypic loss of MHC-II induced specifically by the virus. We have also previously shown that the extent of DC infection depends on cell status because activated DCs become refractory to MCMV infection.24 Here, we show that the combination of reduced DC numbers, reduced infection of DCs, and suboptimal costimulation during GVHD greatly diminished the capacity to generate protective CD8+ T-cell responses.
CMV-specific T cells can be recovered from patient lymphocytes and expanded in vitro in the presence of APC, IL-2, and appropriate antigen.38 Our observations in relation to the defects in DC function in GVHD explain why adoptive T-cell transfer is successful, and predict that interventions that fail to address DC defects are unlikely to improve the control of CMV infection. Thus, one would predict that the provision of IL-2 alone in vivo, for example, would fail to circumvent the primary defect in DCs and be ineffective.
In addition to defects in DC priming, we also observed increased expression of PD1 on CD8+ T cells, including virus-specific IE1+CD8+ T cells, suggestive of T-cell exhaustion. Moreover, expansion of IE1+CD8+ T cells by peptide and DCs ex vivo required exogenous IL-2, also consistent with T-cell exhaustion. It is not clear whether IE1+CD8+ T cells become exhausted before or after viral recognition; however, we observed that a proportion of naive, antigen-specific CD8+ T cells transferred at the time of transplant acquired PD1 prior to exposure to their cognate antigen (R.D.K., G.R.H., and M.A.D.-E., unpublished data). Thus, it is possible that GVHD induces exhaustion, adding another obstacle to the priming of an antiviral T-cell response. Alternatively, IE1+CD8+ T cells may become exhausted as a consequence of suboptimal viral antigen presentation, as reported for some chronic viral infections.39 It is worth noting that although DCs are essential for initiating anti-CMV immunity from naive T cells, it remains possible that additional intrinsic T-cell defects also coexist.
Adoptive transfer of CMV-specific T cells purified and/or expanded from seropositive donors has proven to be an effective therapy for CMV reactivation and disease.14,40 This concept has been advanced by recent studies showing that administration of virus-specific T cells reduces the need for antiviral pharmacotherapy.11 Here, we show that polyclonal antigen-experienced T cells from CMV immune donors bypass the defects in DC priming and protect from CMV disease in a GVHD setting. In a nonhuman primate model, antigen-specific CD8+ T-cell clones derived from central memory T cells showed preferential longevity after adoptive transfer, compared with effector memory T cells.41 Our study did not address this point specifically because a bulk polyclonal population of freshly isolated T cells transferred at the time of transplant conferred maximal protection without a requirement to generate antigen-specific CD8+ T-cell clones. However, it must be noted that although the CMV-specific memory T cells within this polyclonal T-cell population effectively controlled CMV, the contaminating naive T cells were also capable of inducing GVHD. Thus, logical clinical approaches to treat CMV infection in patients with GVHD should focus on comparing the transfer of pathogen-specific memory T cells expanded by in vitro culture with appropriate antigen, vs the transfer of naive T-cell–depleted memory T cells, as recently described by Bleakley et al.42 The longevity of these adoptively transferred T cells is an important clinical consideration and future studies should address this issue.
Most current clinical protocols focus on managing CMV disease with preemptive antiviral therapy administered at first evidence of active infection. Our studies clearly show that during a primary infection, GVHD renders the recipient susceptible to viral pathology; thus, preventing and/or reducing the extent of GVHD should be a clinical priority, preferably while preserving the efficacy of graft-versus-leukemia responses. Furthermore, clinical approaches focused on overcoming DC defects should be explored. A recent trial testing the efficacy of DCs loaded with HCMV peptide demonstrated that this approach could induce or improve HCMV-specific CD8+ T-cell responses and afford long-term control of viral infection.43 Interestingly, it seems that this approach might induce HCMV-specific CD8+ T-cell responses even in settings of low T-cell precursor frequencies (eg, in recipients of seronegative donors). Our studies showing the efficacy of adoptively transferred antigen-specific naive CD8+ T cells indicate that precursor frequencies are a key determinant of effective antiviral T-cell responses. The critical role of antiviral T-cell precursor frequencies would explain why seropositive recipients of grafts from seronegative donors are at the highest risk of CMV disease. Interestingly, this also suggests that the viral antigen presentation requirements are quantitatively and qualitatively less stringent as T-cell precursor frequencies increase.
The timing of adoptive T-cell therapy has until now remained controversial because the infusion of T cells could potentially exacerbate GVHD. Furthermore, given that an effective T-cell response cannot be generated from endogenous donor CD8+ T cells during GVHD, it has been postulated that antiviral T cells transferred early posttransplant might be eliminated or rendered unresponsive by GVHD, thereby negating (or at least reducing) any beneficial antiviral effects. Recent clinical studies have shown that infusion of CMV-specific T cells early posttransplant does not appear to increase GVHD, but reduces the requirement for CMV-directed pharmacotherapy.11 Our adoptive transfer studies in a controlled model system support these findings and demonstrate that there is no difference in the efficacy of antiviral CD8+ T cells transferred at transplant or just before infection. The ability of virus-specific T cells to provide protection may, however, be attenuated by concurrent immune suppression.44
In conclusion, the current study provides important information about the mechanisms that lead to CMV disease in GVHD and defines the cellular pathways that are compromised, thus providing new insights for the development of optimal therapeutic strategies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Slavica Pervan for expert histology assistance.
This work was supported by fellowships and grants from the National Health and Medical Research Council of Australia (NHMRC) and a Cancer Council WA grant.
M.E.W. holds a Brian King Fellowship from the Lions Save Sight Foundation. G.R.H. is an NHMRC Australia Fellow. M.A.D.-E. is an NHMRC Principal Research Fellow.
Authorship
Contribution: M.E.W. designed and performed experiments, analyzed and interpreted data, and cowrote the manuscript; P.F. performed experiments, analyzed and interpreted data, and critically read the manuscript; R.D.K., I.S.S., and V.V. performed experiments, assisted with data analysis, and critically read the manuscript; G.M. and A.D.C. performed the histologic evaluation of GVHD and CMV pathology; S.-K.T. assisted with data analysis and interpretation and assisted with writing the manuscript; C.E.A. designed and performed experiments, analyzed and interpreted the data, and assisted with writing the manuscript; and G.R.H. and M.A.D.-E. conceived the project, designed the experiments, analyzed and interpreted data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mariapia A Degli-Esposti, Lions Eye Institute, 2 Verdun St, Nedlands, Western Australia 6009, Australia; e-mail: mariapia@lei.org.au.
References
Author notes
G.R.H. and M.A.D.-E. contributed equally to this work.