Key Points
The F-BAR protein PACSIN2 associates with the initiating demarcation membrane system in megakaryocytes.
FlnA binding to the PACSIN2 F-BAR domain regulates membrane tubulation in megakaryocytes, platelets, and in vitro.
Abstract
Bin-Amphiphysin-Rvs (BAR) and Fes-CIP4 homology BAR (F-BAR) proteins generate tubular membrane invaginations reminiscent of the megakaryocyte (MK) demarcation membrane system (DMS), which provides membranes necessary for future platelets. The F-BAR protein PACSIN2 is one of the most abundant BAR/F-BAR proteins in platelets and the only one reported to interact with the cytoskeletal and scaffold protein filamin A (FlnA), an essential regulator of platelet formation and function. The FlnA-PACSIN2 interaction was therefore investigated in MKs and platelets. PACSIN2 associated with FlnA in human platelets. The interaction required FlnA immunoglobulin-like repeat 20 and the tip of PACSIN2 F-BAR domain and enhanced PACSIN2 F-BAR domain membrane tubulation in vitro. Most human and wild-type mouse platelets had 1 to 2 distinct PACSIN2 foci associated with cell membrane GPIbα, whereas Flna-null platelets had 0 to 4 or more foci. Endogenous PACSIN2 and transfected enhanced green fluorescent protein-PACSIN2 were concentrated in midstage wild-type mouse MKs in a well-defined invagination of the plasma membrane reminiscent of the initiating DMS and dispersed in the absence of FlnA binding. The DMS appeared less well defined, and platelet territories were not readily visualized in Flna-null MKs. We conclude that the FlnA-PACSIN2 interaction regulates membrane tubulation in MKs and platelets and likely contributes to DMS formation.
Introduction
Blood platelets are produced in the bone marrow by megakaryocytes (MKs) in a process that requires extensive membrane rearrangements. During MK maturation, the demarcation membrane system (DMS) forms as a surface-connected membrane extension that invaginates into the cell body and elaborates to provide membranes for future platelets.1-3 In platelets, the open canalicular system (OCS) forms multiple interconnections between cell surfaces and serves as a membrane reservoir during platelet spreading following activation.4 The MK DMS and platelet OCS share structural similarities, but little is known about the proteins and mechanisms responsible for their formation.
Bin-Amphiphysin-Rvs (BAR) and Fes-CIP4 homology BAR (F-BAR) proteins self-associate into dimers through coiled-coil interaction, bind negatively charged phospholipids on the plasma membrane cytoplasmic surface, and polymerize to generate tubular invaginations reminiscent of the MK DMS and platelet OCS.5-11 The adaptor protein PACSIN2 (also called syndapin 2, FAP52) is one of the most abundant BAR/F-BAR proteins in human and mouse platelets.12,13 PACSIN2 has been implicated in a wide range of cellular functions including cell adhesion, spreading, and migration14-17 ; receptor internalization18,19 ; and caveolae biogenesis.20-22 PACSIN2 is composed of an N-terminal F-BAR domain, 3 central Asn-Pro-Phe motifs, which are docking sites for endocytic Eps15 homology domain proteins,23 and a C-terminal Src homology 3 domain that interacts with actin regulatory and endocytic proteins, such as N-WASP and dynamin 2,24,25 expression of which is required for normal MK development.26
PACSIN2 is the only BAR/F-BAR protein reported to associate and/or colocalize with the cytoskeletal and scaffold protein filamin A (FlnA),14-16 an essential regulator of platelet formation and function.27 Heterozygous mutations in the X-linked FLNA gene in humans have been associated with altered DMS formation, giant platelets, and bleeding tendency,28,29 and a mutation in the PRKACG gene encoding the γ regulatory subunit of protein kinase A has been associated with decreased platelet FlnA expression and consequent macrothrombocytopenia in humans.30 We previously showed that MK-specific Flna deletion in mice results in severe macrothrombocytopenia and prolonged bleeding due to premature release of large, fragile, and poorly functional platelets.31,32 Here we investigated PACSIN2 expression and localization in MKs and platelets and its regulation by FlnA.
Methods
Platelet preparation
Anticoagulated blood was obtained from volunteers by venipuncture. Approval was obtained from the Institutional Review Board of Brigham and Women's Hospital, and informed consent was approved according to the Declaration of Helsinki. Anticoagulated blood was collected from C57BL/6 Flnafl/fl and Flnafl/flPf4-Cre mice by retro-orbital plexus bleeding.32 Mice were treated according to the National Institutes of Health and Boston Children’s Hospital Animal Care and Use Committee guidelines. Human and mouse platelets were prepared as previously described.26,31-34
Immunoblot analysis
Platelets were lysed at 4°C in 1% Nonidet P-40, 150 mM NaCl, 50 mM Tris, pH 7.4, containing 1 mM EGTA, 1 mM Na3VO4, and Complete protease inhibitor cocktail (Roche).26,31,32 For immunoprecipitation studies, lysates were centrifuged at 14 000g for 10 minutes at 4°C. Soluble fractions were incubated with antibodies directed against PACSIN2 (Abcam or Sigma-Aldrich), FlnA (Abcam), or control immunoglobulin (Ig)G (Santa Cruz Biotechnology) for 2 hours at 4°C, followed by incubation with protein G-conjugated Sepharose beads (GE Healthcare) for 1 hour at 4°C.31 Platelet lysates and immune complexes were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis.
For cytoskeletal fraction studies, platelets were lysed in 0.1% Triton X-100, 60 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10 mM EGTA, and 2 mM MgCl2, pH 6.9, containing Complete protease inhibitor cocktail and 2 µM phalloidin (PHEM buffer).33,34 Cytoskeletons were isolated by centrifugation at 100 000g for 30 minutes at 4°C in a Beckman Optima TL ultracentrifuge (Beckman Coulter). Triton X-100 insoluble and soluble fractions were resolved by SDS-PAGE and immunoblot analysis.
PACSIN2 and FlnA constructs
Glutathione S-transferase (GTS)-PACSIN2 F-BAR domain (residues 1-342), His-FlnA, and enhanced green fluorescent protein (EGFP)-FlnA constructs were produced as described.35,36 GST-PACSIN2 F-BAR domain mutant P180A was generated using the QuickChange II XL site-directed mutagenesis kit (Agilent Technologies). Primers used were 5′-CAGCAAGGCAGATGCATCCCTCAACCC-3′ (forward) and 5′-GGGTTGAGGGATGCATCTGCCTTGCTG-3′ (reverse).
PACSIN2 (residues 1-486) was subcloned into murine stem cell virus (MSCV) with EGFP at the C terminus using the InFusion PCR cloning kit (Clontech). Primers used were 5′-GAATTAGATCACCGGTATGTCTGTCACCTACGATGACTCT-3′ (forward) and 5′-CATGGTGGCGACCGGTTTCTGGATAGCCTCGACATAGTTG-3′ (reverse). MSCV-EGFP-PACSIN2 P180A was generated by site-directed mutagenesis as above. MSCV-EGFP-PACSIN2 wild type (WT) and P180A were cotransfected with pCL helper plasmid into 50% to 70% confluent 293T cells using Fugene-6 (Roche).37 Medium was changed 24 hours later, and viral supernatants were harvested at 72 hours. Medium was filtered (0.45 μm), used immediately, or stored at –80°C.
Mapping of the FlnA-PACSIN2 interface
GST-PACSIN2 F-BAR domain WT or P180A (50 nM) was incubated with His-FlnA constructs (500 nM) for 1 hour at 4°C in 1% Nonidet P-40, 150 mM NaCl, and 50 mM Tris, pH 7.4.31 Complexes were pulled down by incubation with glutathione Sepharose beads (GE Healthcare). Complexes were resolved by SDS-PAGE and immunoblot analysis. For rotary shadowing electron microscopy, GST-PACSIN2 F-BAR domain and/or His-FlnA were sprayed on mica in 50% glycerol, dried under vacuum, and metal cast with 1 nm of platinum at 6° with rotation and 3.5 nm of carbon at 90° without rotation.
Membrane tubulation assay
Liposomes were formed using equal parts of phosphatidylcholine (PC) and phosphatidylserine (PS) or phosphatidylinositol (PI) (Sigma-Aldrich). Lipids were mixed at 10 mg/mL in chloroform, and solvent was evaporated under nitrogen on ice and then dried under vacuum for 30 minutes. Lipids were hydrated in 0.25 M sucrose at 50°C for 2 hours. The solution was clarified by centrifugation at 10 000g for 10 minutes and stored at −20°C. Thawed lipids were diluted to 0.2 mg/mL in phosphate-buffered saline (PBS) in the presence or absence of GST-PACSIN2 F-BAR domain WT or P180A, His-FlnA, or EGFP-FlnA constructs. Tubulation capacity was assessed in the electron microscope after negative staining of protein-liposomes solutions with 1% uranyl acetate or in a fluorescent microscope.
Confocal microscopy
Preparation of platelets for immunofluorescence analysis was conducted as previously described.26,38 Platelet preparations were rinsed, blocked, and hybridized under permeabilizing conditions with combinations of primary antibodies that included rabbit anti-PACSIN2 (Abcam), mouse anti-β-tubulin (Sigma-Aldrich), rat anti-GPIbα (Emfret), rat anti-GPIIb (eBioscience), and goat anti-P-selectin (Santa Cruz Biotechnology). After washing, preparations were counterstained with fluorescently labeled secondary antibodies (Invitrogen), washed, postfixed, and mounted with fluorescent medium.
Platelet preparations were imaged via laser fluorescence confocal spinning-disk microscopy (SDM) with a ×63/1.4NA oil-immersion objective on an Olympus 1X81 inverted fluorescence microscope equipped with an Improvision Piezo focus drive (250-nm stepping), ×1.5 magnification lens (Spectral Applied Research), separate diode-pumped solid state laser lines, a spinning disk confocal scan head (Quorum Technologies), and a Hamamatsu EM Back-Thinned EM-CCD camera (512 × 512 pixels). Volocity (version 6.0+) software was used for image acquisition and subsequent processing.
Platelet and MK preparations were imaged via super-resolution structured illumination microscopy (SIM) using a Zeiss Elyra PS1 microscope (Axio Observer Z1 core) and a ×63/1.4NA oil-immersion objective with ×1.6 optovar. The system is equipped with an Andor iXon3 885 detector, 405-, 488-, 561-, and 640-nm laser lines, Zeiss motorized XY stage, and Z-piezo focus. Acquisition control and SIM image processing were done with Zeiss Zen 2012 software; 3-dimensional rendered volume images and movies were created from SIM files using Imaris 7.7.2 ×64 software. Images were exported to Adobe Photoshop for final presentation.
Mouse bone marrow MK preparation and retroviral production
Mouse bone marrow MKs were prepared as previously described.26,39 Flushed bone marrow cells were cultured in 2.6% serum-supplemented StemPro-34 medium (Gibco) with 2 mM l-glutamine, penicillin/streptomycin, and 50 ng/mL recombinant stem cell factor (R&D Systems) for 2 days. Cells were cultured for 2 days in the presence of 50 ng/mL stem cell factor and 50 ng/mL recombinant thrombopoietin (TPO; R&D Systems), and for 2 days in the presence of TPO only. On day 4 of culture, cells were exposed to MSCV-EGFP-PACSIN2 WT and P180A retroviral supernatants in the presence of 8 μg/mL polybrene (Sigma-Aldrich) by centrifugation at 800g for 90 minutes and incubation at 37°C for 90 minutes. Cells were then cultured in fresh medium and TPO for 2 days before isolation of advanced MKs over a bovine serum albumin step-gradient for further analysis. Mature MKs were fixed in 4% paraformaldehyde and centrifuged onto poly-l-lysine-coated coverslips. For SIM imaging experiments, WT mouse MKs were cultured and prepared according to methods previously described,38 where MKs were cultured on Matrigel and then fixed and stained without being cytospun on coverslips.
Transmission electron microscopy
Bone marrow cells were obtained by flushing mouse femurs with 2.5% glutaraldehyde in phosphate-buffered saline; after overnight fixation, they were postfixed with 2% osmium tetroxide in H2O for 1 hour and dehydrated in a graded acetone series before embedding in Epon-Araldite. Thin sections were cut and stained with uranyl acetate and lead citrate. Grids were examined with a JEOL JEM-1011 electron microscope at 80 kV. Images were captured with a side-mounted Advantage HR CCD camera (Advanced Microscopy Techniques).26,38
Statistical analysis
All experiments were performed at least in triplicate. Results were analyzed with the Student t test using Prism software (GraphPad). Differences were considered significant when P < .05.
Results
PACSIN2 expression in platelets
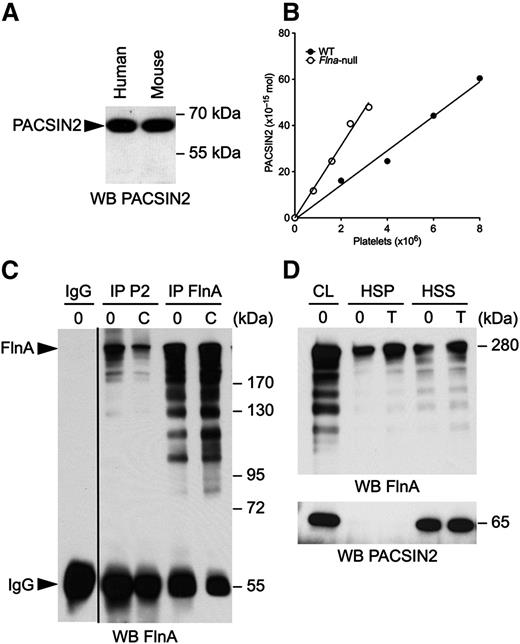
Human and mouse platelet lysates were subjected to SDS-PAGE and probed for PACSIN2 (Figure 1A). PACSIN2 was detected as a single band of 65 kDa in human and mouse platelets, indicating that platelets expressed the long PACSIN2 isoform of 486 residues but not its shorter splicing variant of 445 residues and 52 kDa.18,40 The amount of PACSIN2 molecules in mouse platelets was determined by densitometric analysis of immunoblots using GST-PACSIN2 full length as standard (Figure 1B). Control mouse platelets contained 7.45 × 10−21 mol PACSIN2, or 2200 PACSIN2 dimers, consistent with a previous estimation.13
PACSIN2 associates with FlnA in platelets. (A) Human and mouse platelet lysates were subjected to SDS-PAGE and probed for PACSIN2. (B) Increasing volumes of WT and Flna-null mouse platelet lysates were compared with known amounts of GST-PACSIN2 and probed for PACSIN2. The graph plots the number of PACSIN2 moles against platelet numbers obtained from 2 immunoblots. The amount of PACSIN2 per platelet was determined as the ratio between the first-order equation slopes best fitting the data. (C) Human platelets were activated or not with 10 µg/mL CRP for 2 minutes at 37°C and lysed, and PACSIN2 (P2) and FlnA were immunoprecipitated. A control rabbit IgG was used as negative control for specificity. Immunoprecipitates were subjected to SDS-PAGE and probed for FlnA. (D) Mouse platelets were activated with 1 U/mL thrombin (T) for 2 minutes at 37°C and lysed in PHEM buffer. Triton X-100 HSS and HSP fractions were collected by centrifugation of platelet lysates at 100 000g, subjected to SDS-PAGE, and probed for PACSIN2 and FlnA. CL, cell lysate.
PACSIN2 associates with FlnA in platelets. (A) Human and mouse platelet lysates were subjected to SDS-PAGE and probed for PACSIN2. (B) Increasing volumes of WT and Flna-null mouse platelet lysates were compared with known amounts of GST-PACSIN2 and probed for PACSIN2. The graph plots the number of PACSIN2 moles against platelet numbers obtained from 2 immunoblots. The amount of PACSIN2 per platelet was determined as the ratio between the first-order equation slopes best fitting the data. (C) Human platelets were activated or not with 10 µg/mL CRP for 2 minutes at 37°C and lysed, and PACSIN2 (P2) and FlnA were immunoprecipitated. A control rabbit IgG was used as negative control for specificity. Immunoprecipitates were subjected to SDS-PAGE and probed for FlnA. (D) Mouse platelets were activated with 1 U/mL thrombin (T) for 2 minutes at 37°C and lysed in PHEM buffer. Triton X-100 HSS and HSP fractions were collected by centrifugation of platelet lysates at 100 000g, subjected to SDS-PAGE, and probed for PACSIN2 and FlnA. CL, cell lysate.
PACSIN2 associates with FlnA in platelets
PACSIN2 associates and/or colocalizes with FlnA in chicken fibroblasts, Xenopus XTC cells, and human carcinoma cells.14-16 Their interaction was therefore investigated in human platelets, both resting and activated with 10 µg/mL of collagen-related peptide (CRP) for 2 minutes. Platelets were lysed, and PACSIN2 and FlnA were immunoprecipitated, subjected to SDS-PAGE, and probed for FlnA (Figure 1C). Both resting and CRP-activated platelet PACSIN2 pulled down FlnA, detected as a band of 280 kDa.
Mouse platelets were activated with 1 U/mL thrombin for 2 minutes and lysed in PHEM buffer. Triton X-100 high-speed soluble (HSS) and pellet (HSP) fractions were collected by centrifugation of platelet lysates at 100 000g, subjected to SDS-PAGE, and probed for PACSIN2 and FlnA. About half of FlnA associated with F-actin, the HSP phase (Figure 1D). PACSIN2 remained in the HSS phase in resting and thrombin-stimulated platelets, indicating that, although PACSIN2 associated with FlnA, it failed to co-sediment with the platelet actin cytoskeleton.
Mapping of the PACSIN2 binding site on FlnA
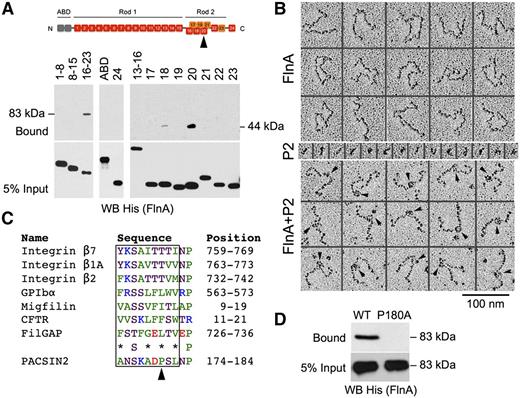
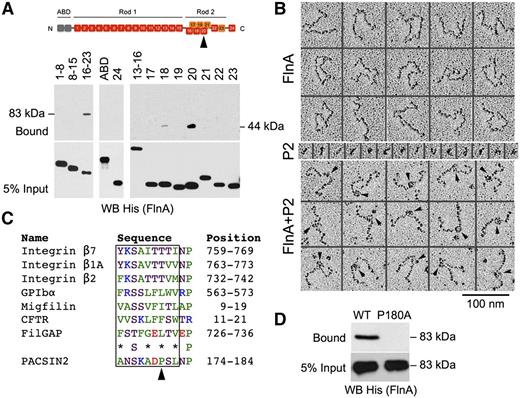
The FlnA-PACSIN2 interface was mapped in vitro using recombinant proteins (Figure 2). FlnA is composed of an N-terminal tandem calponin-homology actin-binding domain (ABD), followed by 24 immunoglobulin-like repeats, which are structurally separated into a linear rod 1 (repeats 1-15), a compact rod 2 (repeats 16-23), and a C-terminal self-association domain (repeat 24).36 GST-PACSIN2 F-BAR domain was incubated with His-tagged constructs containing FlnA ABD, repeats 1 to 8, 8 to 15, 16 to 23, or 24, because FlnA interacts with the PACSIN2 F-BAR domain.13,14 GST-PACSIN2 F-BAR domain pulled down FlnA repeats 16 to 23 (Figure 2A), indicating that PACSIN2 associated with the compact rod 2, a region that also binds GPIbα and integrin β1.41,42 No binding of FlnA ABD, repeats 1 to 8, 8 to 15, or 24 to GST-PACSIN2 F-BAR domain was detected. The binding site was further dissected using His-tagged FlnA repeats 13 to 16 or single repeats 17 to 23, which identified FlnA repeat 20 as the PACSIN2 binding site. Weaker binding to repeat 18 was also observed.
Mapping of the FlnA-PACSIN2 interface. (A) GST-PACSIN2 F-BAR domain was incubated with His-tagged FlnA recombinant truncates as indicated. Complexes were pulled down with glutathione Sepharose beads, subjected to SDS-PAGE, and probed for His (FlnA). FlnA rod 2 (repeats 16-23; 83 kDa) and repeat 20 (44 kDa) were collected with glutathione Sepharose beads. FlnA domains are shown on the top of the panel. The arrowhead points to repeat 20. (B) Electron microscopy of FlnA and GST-PACSIN2 F-BAR domain (P2) observed by rotary shadowing. PACSIN2 binds ∼15 nm from the FlnA self-association site. Arrowheads point to PACSIN2 bound along FlnA molecules. (C) FlnA-binding motif of PACSIN2 compared with that of known FlnA partners. Protein names, amino acid sequences, and positions are indicated. Nonpolar residues are colored green, neutral are magenta, basic are blue, and acidic are red. *Nonpolar or neutral residue. The arrowhead points to PACSIN2 Pro180. (D) GST-PACSIN2 F-BAR domain WT or P180A was incubated with His-FlnA repeats 16 to 23. Complexes were pulled down with glutathione Sepharose beads, subjected to SDS-PAGE, and probed for His (FlnA).
Mapping of the FlnA-PACSIN2 interface. (A) GST-PACSIN2 F-BAR domain was incubated with His-tagged FlnA recombinant truncates as indicated. Complexes were pulled down with glutathione Sepharose beads, subjected to SDS-PAGE, and probed for His (FlnA). FlnA rod 2 (repeats 16-23; 83 kDa) and repeat 20 (44 kDa) were collected with glutathione Sepharose beads. FlnA domains are shown on the top of the panel. The arrowhead points to repeat 20. (B) Electron microscopy of FlnA and GST-PACSIN2 F-BAR domain (P2) observed by rotary shadowing. PACSIN2 binds ∼15 nm from the FlnA self-association site. Arrowheads point to PACSIN2 bound along FlnA molecules. (C) FlnA-binding motif of PACSIN2 compared with that of known FlnA partners. Protein names, amino acid sequences, and positions are indicated. Nonpolar residues are colored green, neutral are magenta, basic are blue, and acidic are red. *Nonpolar or neutral residue. The arrowhead points to PACSIN2 Pro180. (D) GST-PACSIN2 F-BAR domain WT or P180A was incubated with His-FlnA repeats 16 to 23. Complexes were pulled down with glutathione Sepharose beads, subjected to SDS-PAGE, and probed for His (FlnA).
The PACSIN2 binding location on FlnA was confirmed in rotary shadowed samples (Figure 2B). Dimeric FlnA molecules are 160 nm in length,36 and PACSIN2 F-BAR domains self-assemble into unique S-shaped dimers, ∼20 nm in length,43-47 which can be visualized in low angle metal cast samples. When FlnA was incubated with GST-PACSIN2 F-BAR domain, the latter could be visualized on FlnA molecules attached 64 ± 5 nm (mean ± standard deviation; n = 33) from the most proximate free FlnA end. Thus, the PACSIN2 F-BAR domain bound close to the predicted location on repeat 20, which is expected to be located ∼67 nm from each free end.36
Mapping of the FlnA binding site on PACSIN2
Proteins binding to FlnA repeats 17 and 21, such as GPIbα and integrin β1, respectively, contain a FlnA-binding motif, a β-strand of 9 residues that inserts into a groove formed by 2 antiparallel β-strands named C and D in the FlnA repeat.41,42 This FlnA-binding motif contains nonpolar or neutral residues at alternate positions and a relatively conserved neutral Ser at position 3.27,48 Analysis of the PACSIN2 F-BAR domain sequence identified a putative FlnA-binding motif at position 174 to 182 (Figure 2C), in a loop located at the tip of the F-BAR domain, between the second and third α-helixes,43-47 and within a region previously described as binding FlnA (residues 146-184).14
One major difference between the putative FlnA-binding motif of PACSIN2 and that of previously characterized FlnA partners is the presence of a Pro at position 180, which excludes formation of a long β-strand. Because the structure of FlnA repeat 20 differs from that of repeats 17 and 21, as a short α-helix precedes its β-strand C,49,50 we hypothesized that PACSIN2 Pro180 forms a necessary kink to avoid the α-helix. A GST-PACSIN2 F-BAR domain mutant P180A was generated to test this hypothesis. Substitution P180A abolished PACSIN2 binding to His-FlnA repeats 16 to 23 (Figure 2D), confirming that the FlnA-binding site was located at the tip of the F-BAR domain.
FlnA binding to the PACSIN2 F-BAR domain enhances membrane tubulation in vitro
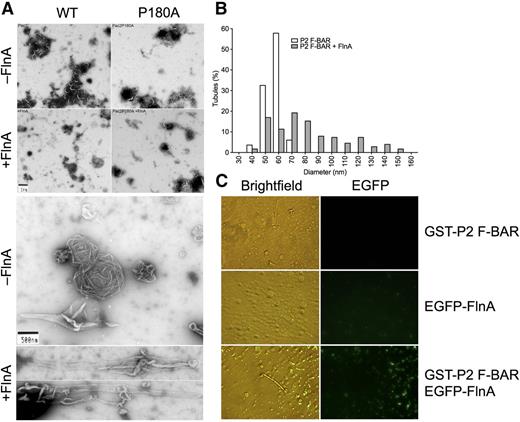
PACSIN2 is a flexible dimer and can generate membrane tubules of low and high curvatures.43,46,47 Similarly, FlnA is a flexible dimer, due to the presence of 2 hinges between repeats 15 and 16 and repeats 23 and 24.36 Further, FlnA binds PACSIN2 near residues 184 to 187, which are necessary for tip-to-tip interaction and membrane tubulation.47 Membrane tubulation induced by GST-PACSIN2 F-BAR domain was therefore assessed in vitro in the presence or absence of His-FlnA (Figure 3).
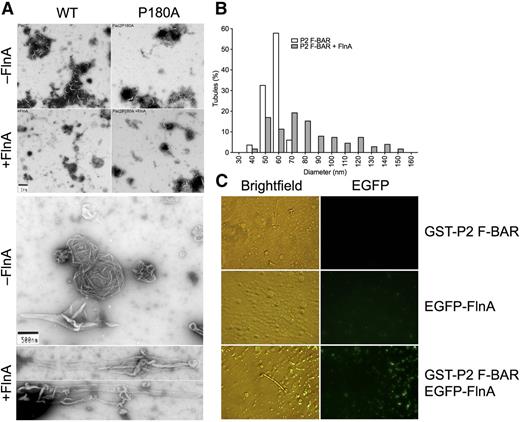
FlnA potentiates PACSIN2 F-BAR domain membrane tubulation in vitro. (A) PC/PS liposomes were incubated with GST-PACSIN2 F-BAR domain WT or P180A in the presence or absence of His-FlnA constructs, as indicated. Membrane tubulation capacity was assessed in the electron microscope. (B) Diameter of membrane tubules generated by GST-PACSIN2 (P2) F-BAR domain in the presence or absence of His-FlnA. (C) PC/PI liposomes were incubated with GST-PACSIN2 (P2) F-BAR domain and/or EGFP-FlnA and observed by light and fluorescence microscopy.
FlnA potentiates PACSIN2 F-BAR domain membrane tubulation in vitro. (A) PC/PS liposomes were incubated with GST-PACSIN2 F-BAR domain WT or P180A in the presence or absence of His-FlnA constructs, as indicated. Membrane tubulation capacity was assessed in the electron microscope. (B) Diameter of membrane tubules generated by GST-PACSIN2 (P2) F-BAR domain in the presence or absence of His-FlnA. (C) PC/PI liposomes were incubated with GST-PACSIN2 (P2) F-BAR domain and/or EGFP-FlnA and observed by light and fluorescence microscopy.
GST-PACSIN2 F-BAR domain generated membrane tubules from PC/PS liposomes with an average diameter of 53 ± 6 nm (mean ± standard deviation; range, 35-67 nm; n = 83; Figure 3A-B), consistent with previous observations.43,46,47 Full-length FlnA and dimerized FlnA repeats 16 to 24 but not dimerized FlnA repeats 1 to 8+24 and 8 to 15+24 accelerated membrane tubulation initiated by GST-PACSIN2 F-BAR domain (Figure 3A-B; Table 1). The presence of full-length FlnA tripled the number of elongated structures found in negatively stained samples and increased the tubule diameter to 77 ± 27 nm (mean ± standard deviation; range, 36-150 nm; n = 177; P < .0001), although a wide distribution was observed, and FlnA modestly affected the tubule length. As control, FlnA alone did not promote tubule formation (Table 1).
GST-PACSIN2 F-BAR mutant P180A generated tubules comparable to those of GST-PACSIN2 F-BAR WT (Figure 3A; Table 1), indicating that the substitution did not affect tip-to-tip interaction and membrane tubulation. However, FlnA failed to enhance membrane tubulation generated by GST-PACSIN2 F-BAR mutant P180A, indicating that FlnA binding to PACSIN2 F-BAR domain was necessary to enhance tubule formation.
Experiments to determine whether FlnA was bound along the tubules generated by GST-PACSIN2 F-BAR domain were routinely negative (data not shown), suggesting that FlnA could no longer bind PACSIN2 once the F-BAR domain dimers polymerized around tubules. To explore this possibility, PC/PI liposomes were tubulated by GST-PACSIN2 F-BAR domain in the light microscope in the presence of recombinant EGFP-FlnA. EGFP-FlnA associated PACSIN2 on small round liposomes, but was dissociated from tubulated lipid regions (Figure 3C). Taken together, the results demonstrate that FlnA enhanced membrane tubulation of GST-PACSIN2 F-BAR domain but dissociated from PACSIN2 during the tubulation process.
PACSIN2 resides at the entrance of platelet membrane invaginations
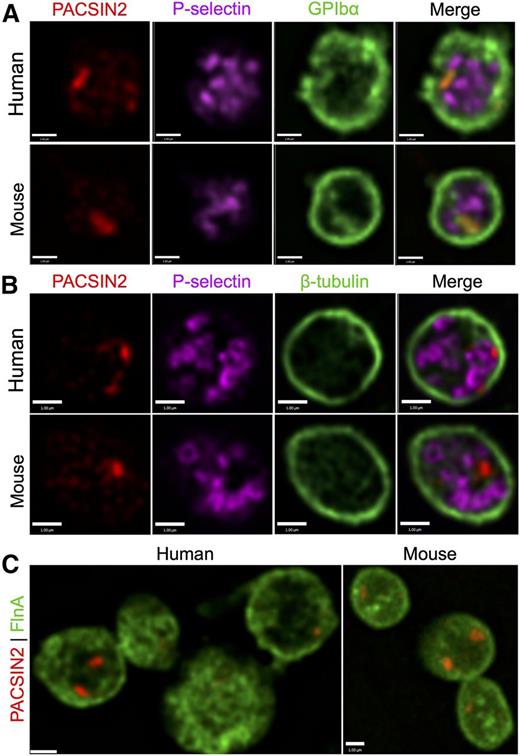
PACSIN2 localization in human and mouse platelets was investigated by high-resolution SDM (Figure 4). PACSIN2 staining was typically confined to 1 to 2 distinct foci, which showed little apparent association with α-granules (P-selectin) or the platelet marginal band (tubulin) (Figure 4A-B), nor with dense granules (CD63), lysosomes (LAMP1), cis-Golgi (GM130), trans-Golgi (TGN46) or F-actin (data not shown). Costaining for PACSIN2 and GPIbα (Figure 4A) indicated localized overlap at locations suggestive of entrances to the platelet OCS or another internal membrane system. Similar localized contact was observed in human platelets between PACSIN2 and FlnA (Figure 4C).
PACSIN2 localization in human and mouse platelets. High-resolution SDM of (A) PACSIN2 (red), P-selectin (magenta), and GPIbα (green) in fixed human and mouse platelets; (B) PACSIN2 (red), P-selectin (magenta), and β-tubulin (green) in fixed human and mouse platelets; and (C) PACSIN2 (red) and FlnA (green) in fixed human and mouse platelets. Most platelets show 1 to 2 distinct concentrated PACSIN2 foci, which may also be distributed throughout the cells. Scale bars represent 1 µm.
PACSIN2 localization in human and mouse platelets. High-resolution SDM of (A) PACSIN2 (red), P-selectin (magenta), and GPIbα (green) in fixed human and mouse platelets; (B) PACSIN2 (red), P-selectin (magenta), and β-tubulin (green) in fixed human and mouse platelets; and (C) PACSIN2 (red) and FlnA (green) in fixed human and mouse platelets. Most platelets show 1 to 2 distinct concentrated PACSIN2 foci, which may also be distributed throughout the cells. Scale bars represent 1 µm.
PACSIN2 localization in platelets requires FlnA
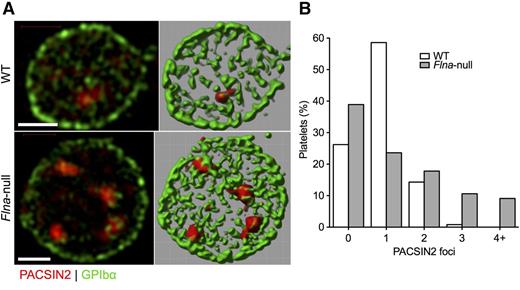
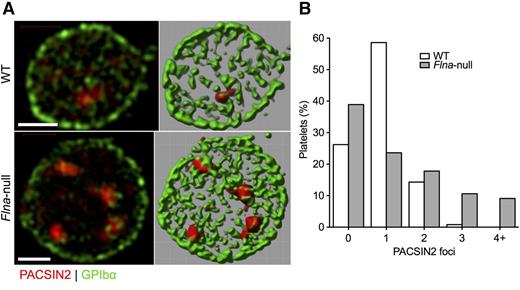
Examination by super-resolution SIM showed that PACSIN2 localization in Flnafl/flPf4-Cre (Flna-null) platelets differed from that observed in control Flnafl/fl platelets (Figure 5A; supplemental Video 1, available on the Blood Web site). Although most WT platelets had a single PACSIN2 focus, the largest group of Flna-null platelets had no definite focus, and many had ≥3 foci (Figure 5B; supplemental Video 2), which was not seen in normal cells. These foci were in apparent contact with the plasma membrane, here labeled for GPIbα.
PACSIN2 localization in WT and Flna-null platelets. (A) Super-resolution SIM of PACSIN2 (red) and GPIbα (green) in typical fixed WT and Flna-null mouse platelets (left). Fields were rendered in Volocity extended focus mode (right). Scale bars represent 1 µm. (B) Fields rendered were scored for the presence of defined PACSIN2 foci at a constant contrast setting. Total platelets scored were 244 WT and 208 Flna null.
PACSIN2 localization in WT and Flna-null platelets. (A) Super-resolution SIM of PACSIN2 (red) and GPIbα (green) in typical fixed WT and Flna-null mouse platelets (left). Fields were rendered in Volocity extended focus mode (right). Scale bars represent 1 µm. (B) Fields rendered were scored for the presence of defined PACSIN2 foci at a constant contrast setting. Total platelets scored were 244 WT and 208 Flna null.
Flna-null platelets contained 15.6 × 10−21 mol PACSIN2, or 4700 PACSIN2 dimers (Figure 1B), a 2.1-fold increase compared with control platelets, consistent with the previously reported increased volume of Flna-null platelets.31,32 PACSIN2 expression levels in Flna-null MKs were similar to those of control MKs (data not shown).
PACSIN2 localization in MKs requires FlnA binding
We analyzed the distribution of PACSIN2 in WT cultured mouse bone marrow MKs by SIM (Figure 6). In midstage MKs, PACSIN2 was confined mostly to a single locus, which showed contact with colabeled CD41 (Figure 6A; supplemental Videos 3 and 4), consistent with plasma membrane invagination and reminiscent of the recently characterized initiating DMS.3 In contrast, in mature MKs, PACSIN2 was observed to be spread throughout the cell in foci similar in size to those observed in platelets (Figure 6B; supplemental Video 5).
PACSIN2 localization in bone marrow MKs requires FlnA. Super-resolution SIM of PACSIN2 (red), CD41 (green), and DAPI (blue) in fixed (A) midstage and (B) mature matrigel-cultured WT mouse MKs. Arrowhead indicates tubule-associated PACSIN2 concentration. Scale bars represent 2 and 3 µm, respectively. (C) Confocal microscopy of PACSIN2 (green) and β-tubulin (red) in fixed cultured WT (upper) and Flna-null mouse (lower) bone marrow MKs. Scale bar represents 20 µm. (D) Confocal microscopy of EGFP-PACSIN2 WT or mutant P180A (green), and CD41 (red) in fixed control and Flna-null cultured cytospun bone marrow MKs, as indicated. Scale bar represents 20 µm. (E) Percentage of transfected MKs with defined EGFP-PACSIN2+ membrane tubulation was scored. Total MKs scored were 46 WT and 18 Flna null.
PACSIN2 localization in bone marrow MKs requires FlnA. Super-resolution SIM of PACSIN2 (red), CD41 (green), and DAPI (blue) in fixed (A) midstage and (B) mature matrigel-cultured WT mouse MKs. Arrowhead indicates tubule-associated PACSIN2 concentration. Scale bars represent 2 and 3 µm, respectively. (C) Confocal microscopy of PACSIN2 (green) and β-tubulin (red) in fixed cultured WT (upper) and Flna-null mouse (lower) bone marrow MKs. Scale bar represents 20 µm. (D) Confocal microscopy of EGFP-PACSIN2 WT or mutant P180A (green), and CD41 (red) in fixed control and Flna-null cultured cytospun bone marrow MKs, as indicated. Scale bar represents 20 µm. (E) Percentage of transfected MKs with defined EGFP-PACSIN2+ membrane tubulation was scored. Total MKs scored were 46 WT and 18 Flna null.
A comparison of PACSIN2 distribution in cultured WT and Flna-null MKs via confocal immunofluorescence microscopy (Figure 6C) showed that, although PACSIN2 appeared to be associated with tubular membrane structures in WT MKs, in the absence of FlnA, it was dispersed throughout the cell. This observation was confirmed by transfection studies (Figure 6D-E). Consistent with mislocalization of endogenous PACSIN2 in Flna-null MKs, transfected EGFP-PACSIN2 was found dispersed throughout Flna-null MKs, with only 22.2% (4 of 18) presenting EGFP+ tubules, compared with 82.6% (19 of 23) of transfected WT MKs. These EGFP+ membrane tubules were connected to the plasma membrane, as shown by CD41 costaining (Figure 6D).
Crosslinking of actin filaments and anchorage of GPIbα to the actin cytoskeleton are altered in Flna-null platelets and MKs.31,32 EGFP-PACSIN2 mutant P180A was therefore generated and transfected into WT MKs to determine whether loss of FlnA binding altered PACSIN2 localization. EGFP-PACSIN2 P180A was indeed observed to be dispersed throughout these cells (Figure 6D), with only 30.4% (7 of 23) presenting EGFP+ tubules (Figure 6E). Taken together, these results indicate that FlnA binding to PACSIN2 regulates membrane tubulation in MKs.
Altered ultrastructure of Flna-null bone marrow MKs
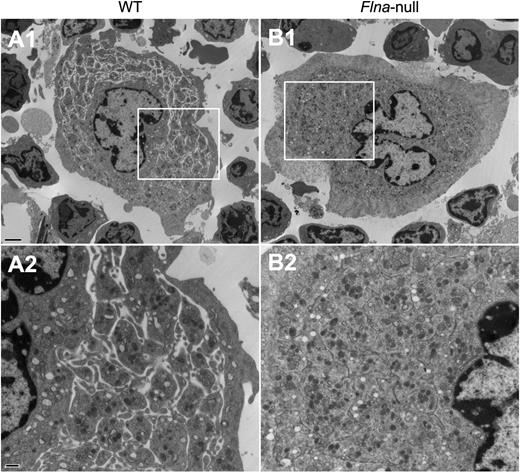
The ultrastructure of bone marrow MKs from control Flnafl/fl and Flnafl/flPf4-Cre mice was examined by transmission electron microscopy, focusing on the DMS, the highly organized intracellular membrane reservoir for future platelets that appears in late mature MKs.1-3 Although the DMS consistently formed normally in control MKs (Figure 7A), it appeared to be less well defined in Flna-null MKs (Figure 7B), where distinct platelet territories were not readily visualized.
Altered ultrastructure of Flna-null bone marrow MKs. Transmission electron microscopy analysis of freshly isolated bone marrow (A) WT and (B) Flna-null MKs. Areas within boxes in micrographs A1 and B1 are shown at higher magnification in A2 and B2, respectively. Scale bars represent 2 µm at ×5000 magnification (upper) and 500 nm at ×15000 magnification (lower).
Altered ultrastructure of Flna-null bone marrow MKs. Transmission electron microscopy analysis of freshly isolated bone marrow (A) WT and (B) Flna-null MKs. Areas within boxes in micrographs A1 and B1 are shown at higher magnification in A2 and B2, respectively. Scale bars represent 2 µm at ×5000 magnification (upper) and 500 nm at ×15000 magnification (lower).
Discussion
The MK DMS and platelet OCS play critical roles and share structural similarities, but little is known about the proteins and developmental mechanisms responsible for their formation and organization. Our results show that FlnA binding to PACSIN2 F-BAR domain regulates membrane tubulation in MKs and platelets and likely contributes to DMS formation.
Previous studies have shown that BAR/F-BAR proteins modulate platelet function. The F-BAR-containing tyrosine kinases Fes and Fer regulate platelet aggregation induced by collagen and ADP,51 and the BAR-containing RhoGAPs oligophrenin 1 and nadrin regulate platelet cytoskeletal rearrangements.52-55 However, these studies focused on properties associated with enzymatic activities of these proteins, independently of their ability to tubulate membranes. Another recent study has shown that mice lacking the F-BAR-containing adaptor protein CIP4 develop mild thrombocytopenia,56 similar to mice lacking WASp,57,58 the protein mutated in Wiskott-Aldrich syndrome. CIP4 is essential for coordinated membrane tubulation and actin cytoskeletal reorganization during endocytosis, as it recruits the small GTPase Cdc42 and the WASp paralog N-WASP to clathrin-coated pits.8 However, how CIP4 regulates platelet production is unclear, as both Cip4-null and Wasp-null MKs produce platelets of normal morphology. Although Cip4-null, but not Wasp-null, bone marrow MKs have defective DMS formation,56,57 no association among CIP4, WASp, and the MK DMS and/or platelet OCS has been described.
The functional interaction between FlnA and PACSIN2 in MKs and platelets is consistent with previous observations.14-16 FlnA appears to be required for correct localization/function of PACSIN2 in MKs, as evidenced by dispersed PACSIN2 staining in Flna-null MKs and in WT MKs where FlnA binding was disrupted via PACSIN2 mutant P180A. The functional consequences of this mistargeting are likely related to the association of PACSIN2 with the recently characterized initiating DMS,3 because super-resolution microscopy analysis of endogenous PACSIN2 and transfected EGFP-PACSIN2 in cultured mouse MKs at midstage maturation revealed an association with well-defined tubular membrane structures connected to the plasma membrane. This observation suggests that PACSIN2 membrane tubulation activity contributes to the membrane invagination that initiates the DMS and possibly supports DMS maturation. Consistent with this hypothesis, we observed DMS and platelet territories in freshly isolated mature Flna-null MKs to be less well defined than in WT MKs, paralleling observations in patients with FLNA mutations.28
The dispersed localization of PACSIN2 observed in mature MKs suggests that it is no longer involved in the formation of tubular structures after the DMS is formed. However, as with MKs, the presence of FlnA appears to be required for targeting of PACSIN2 within platelets, because microscopic examination shows PACSIN2 to be limited to 1 to 2 distinct caveolae-like foci in human and WT mouse platelets, whereas Flna-null mouse platelets had indeterminate or multiple foci. Although the precise function of these PACSIN2 foci remains to be determined, their localization near the platelet plasma membrane, as indicated by association with GPIbα, suggests a role in the formation and/or function of the platelet OCS.
Our results map the FlnA-PACSIN2 interface to FlnA repeat 20 in the compact rod 2 and a novel FlnA-binding motif (residues 174-182) at the tip of PACSIN2 F-BAR domain and demonstrate that PACSIN2 Pro180 is critical for FlnA binding in vitro and in cultured bone marrow MKs. Interestingly, Pro180 is unique to PACSIN2, perhaps explaining why PACSIN2 is the only BAR/F-BAR protein reported to associate with FlnA. For example, neuron-specific PACSIN1 lacks Pro180 and instead contains a highly conserved regulatory phosphorylation site on Thr181, absent in PACSIN2.59 Nikki et al have reported that the PACSIN2 binding site in FlnA resides within repeats 15 to 16,14 whereas no association between PACSIN2 and FlnA repeats 13 to 16 was observed in our experiments. One reason for this discrepancy might be the use of bacterially expressed GST-FlnA truncates in the original study, which does not exclude binding to repeat 20, compared with insect cell expressed His-tagged FlnA repeats here.
Compared with most F-BAR proteins, which only generate membrane tubules of low curvatures, PACSIN2 and its paralog PACSIN1 generate membrane tubules of low and high curvatures, due to the unique S-shape of their F-BAR domain and the presence of a flexible and hydrophobic wedge loop located within the second α-helix.43-47,59,60 PACSIN1 Thr181 phosphorylation, at the N-cap of the third α-helix, negatively regulates membrane tubulation in neurons.59 Our results identify FlnA binding to PACSIN2 F-BAR domain as a novel phosphorylation-independent regulatory mechanism for membrane tubulation. Interestingly, muscle-specific PACSIN3 lacks both the PACSIN2 FlnA-binding motif (residues 174-182) and the PACSIN1 phosphorylation site (Thr181) and only generates membrane tubules of low curvatures, due to a more rigid wedge loop within the second α-helix of its F-BAR domain.46 PACSIN1 and PACSIN3 were not detected in platelets (data not shown), consistent with mRNA and protein profiling.12,13
The FlnA-binding site is located near PACSIN2 residues 184 to 187, which are necessary for tip-to-tip interaction and membrane tubulation.47 Experiments to determine whether FlnA was bound along membrane tubules were routinely negative, suggesting that FlnA molecules initially bind to PACSIN2 F-BAR domains to catalyze membrane tubulation but dissociate from PACSIN2 once membrane tubules elongate. This would account for our observations that (1) FlnA was not found on maximally tubulated vesicles in vitro; (2) total platelet PACSIN2 was released from the cytoskeleton following lysis with Triton X-100; and (3) PACSIN2 showed little colocalization with more abundant FlnA, CD41, or GPIbα in MKs and platelets. Our results suggest that the function of FlnA binding to PACSIN2 is not to attach membrane tubules to the actin cytoskeleton but to move PACSIN2 between internal membrane sites, contributing to the organization of MK and platelet internal membrane systems.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Terese Jönsson, Julia Sjödin, and Ling Li for technical assistance; Dr Hugh Kim and Mike Woodside for help with microscopy; and Drs Jari Ylänne and Markus Bender for helpful discussions.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL089224, HL107146, HL059561, HL104145, HL036153, and HL086655; Canadian Institutes of Health Research grant MOP-119450; and the Brigham Research Institute Fund to Sustain Research Excellence (to H.F.).
Authorship
Contribution: All authors designed and performed research, analyzed, and interpreted the data and contributed to the writing of the manuscript; and H.F. designed the study and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.J.B. is Department of Biotechnology, University of Rijeka, Rijeka, Croatia.
The current affiliation for W.S. is Department of Anatomy, Faculty of Science, Mahidol University, Bangkok, Thailand.
Correspondence: Hervé Falet, Division of Hematology, Brigham and Women's Hospital, One Blackfan Circle, Karp 6, Boston, MA 02115, USA; e-mail: hfalet@rics.bwh.harvard.edu.
References
Author notes
A.J.B. and F.G.P. contributed equally to this work.