Key Points
MYD88 mutation analysis significantly improves the detection rate of vitreoretinal B-cell lymphoma.
The high frequency of MYD88 mutations in primary VRL provides further evidence that VRL and primary CNS lymphoma represent the same entity.
Abstract
Vitreoretinal diffuse large B-cell lymphoma is a rare disorder, occurring as primary ocular disease or as secondary involvement by primary central nervous system lymphoma. It is usually diagnosed by cytologic, immunocytochemical, and molecular examination of vitreous aspirates. However, distinguishing vitreoretinal diffuse large B-cell lymphoma from uveitis remains difficult, and clonality analysis may be either unsuccessful or misleading. Diffuse large B-cell lymphoma arising in immune-privileged sites (eg, the central nervous system) shows a high frequency of MYD88 mutations. Therefore, we retrospectively assessed the frequency of MYD88 mutations in vitreoretinal lymphoma (VRL) and their diagnostic potential in 75 vitrectomy samples of 69 patients, and validated our results in a separate cohort (n = 21). MYD88 mutations were identified in 20 of 29 (69%) clinically, histologically, and molecularly confirmed VRL, including 6 cases of the test cohort initially diagnosed as reactive (3/6) or suspicious (3/6) for lymphoma. MYD88 mutations, especially L265P, are very frequent in VRL and their detection significantly improves the diagnostic yield of vitrectomy specimens.
Introduction
Vitreoretinal lymphoma (VRL), known previously as “primary intraocular lymphoma,” is a rare ocular malignancy with overall poor prognosis, manifesting most commonly in the retina and vitreous body. Most cases are classified as diffuse large B-cell lymphomas (DLBCLs) and represent either primary VRL or secondary ocular involvement by primary central nervous system lymphoma (PCNSL).1-5 VRLs are highly aggressive tumors that need to be distinguished from the second most common intraocular lymphoma, low-grade choroidal extranodal marginal zone B-cell lymphomas, which are not associated with central nervous system (CNS) disease but which do occasionally extend extraocularly.2
VRL is usually diagnosed by cytologic, immunocytochemical, and molecular examination of vitreous body aspirates. However, diagnosis is often challenging because of the limited material sent for analysis. Clinically, VRL frequently resembles uveitis6 and cytologic diagnosis is difficult because of low numbers of neoplastic cells, a commonly high admixture of non-neoplastic lymphocytes, and profound degenerative changes.2
Detection of monoclonal rearrangements of the immunoglobulin heavy chain (IGH) genes provides support for a diagnosis of VRL,7 but clonality analysis may be misleading because of pseudoclonal/oligoclonal patterns resulting from low cellularity or benign B-cell clones in immunologic disorders.8,9 Additional helpful diagnostic procedures include flow cytometry and the identification of an elevated IL-10/IL-6 ratio, but the small amount of vitreous fluid remains the limiting factor.10-15 PNCSL, which is considered as closely related to primary VRL, is usually of postgerminal-center origin (activated B-cell type) as defined by gene expression and mutational profile.1,16 A feature of DLBCL arising in immune-privileged sites (eg, the CNS) is a high frequency of MYD88 mutations (mostly L265P, rarely codon 103 and 143), found in as much as 75% of PCNSL.17-19 Given the lack of mutational data and the diagnostic difficulties in VRL, the aim of our study was to assess the frequency of MYD88 mutations in VRL and to investigate their diagnostic potential in a large series of vitrectomy specimens.
Study design
Patients
Seventy-five diagnostic vitrectomy samples of 69 patients with available archival DNA obtained consecutively between January 2008 and August 2014 and analyzed at the Institute of Pathology, University Hospital Tübingen, were included in this study. All samples had been examined by standard cytology and immunocytochemistry for B- and T-cell markers, as well as clonality analysis for the presence of IGH rearrangements, unless cytomorphology had revealed evidence for infectious endophthalmitis. As a validation cohort, 21 archival DNA samples derived from vitrectomy specimens previously diagnosed at the Department of Molecular & Clinical Cancer Medicine, University of Liverpool, were analyzed in comparison.
All available data including clinical charts, ophthalmic findings (Figure 1A), cytology and clonality analyses, and follow-up information were jointly reviewed for all patients. After review, a joint consensus diagnosis was rendered and correlated with MYD88 mutation status.
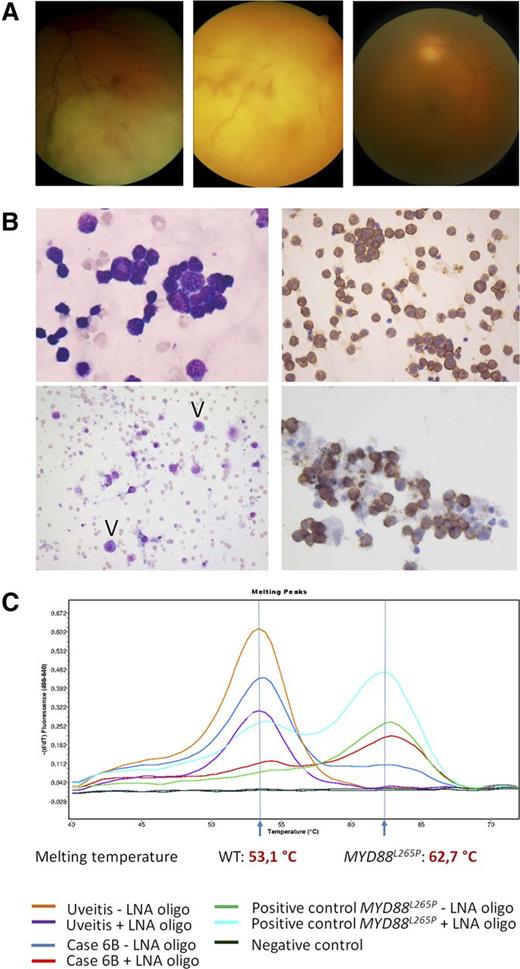
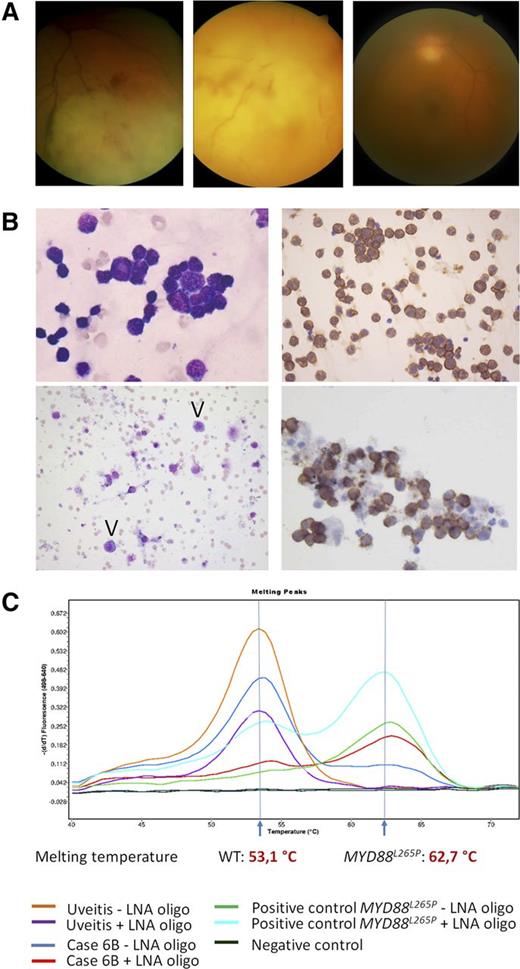
Clinical, cytologic, immunohistochemical, and molecular findings in VRL. (A) Representative fundus photographs of 3 VRL patients: VRL with retinal and choroidal infiltration (left), with massive infiltration at rear pole (middle) and with vitreous infiltration only (right). (B) Upper left: PVRL, 49-year-old woman, MYD88L265P. Highly cellular vitreous body aspirate with numerous atypical lymphocytes (May-Grünwald-Giemsa staining, left). Virtually all cells stain for CD20 (immunoperoxidase, right). Lower left: PVRL, 63-year-old woman, MYD88L265P. The aspirate shows rare atypical cells with basophilic cytoplasm (arrowheads, May-Grünwald-Giemsa staining). Clonality analysis was noncontributory and the case was considered as nondiagnostic for PVRL. Lower right: PVRL, 70-year-old woman. MYD88L265P, monoclonal IGH rearrangement. CD20 stain. All images taken at original magnification ×400. (C) Representative example of MYD88 codon 265 locked nucleic acid (LNA)-clamped, allele-specific PCR and melting curve analysis. One case with final diagnosis uveitis shows the WT curve with the melting peak at 62.7°C, whereas case 6B, which initially was considered reactive, turned out to be MYD88L265P-mutated, melting at 53.1°C.
Clinical, cytologic, immunohistochemical, and molecular findings in VRL. (A) Representative fundus photographs of 3 VRL patients: VRL with retinal and choroidal infiltration (left), with massive infiltration at rear pole (middle) and with vitreous infiltration only (right). (B) Upper left: PVRL, 49-year-old woman, MYD88L265P. Highly cellular vitreous body aspirate with numerous atypical lymphocytes (May-Grünwald-Giemsa staining, left). Virtually all cells stain for CD20 (immunoperoxidase, right). Lower left: PVRL, 63-year-old woman, MYD88L265P. The aspirate shows rare atypical cells with basophilic cytoplasm (arrowheads, May-Grünwald-Giemsa staining). Clonality analysis was noncontributory and the case was considered as nondiagnostic for PVRL. Lower right: PVRL, 70-year-old woman. MYD88L265P, monoclonal IGH rearrangement. CD20 stain. All images taken at original magnification ×400. (C) Representative example of MYD88 codon 265 locked nucleic acid (LNA)-clamped, allele-specific PCR and melting curve analysis. One case with final diagnosis uveitis shows the WT curve with the melting peak at 62.7°C, whereas case 6B, which initially was considered reactive, turned out to be MYD88L265P-mutated, melting at 53.1°C.
Cytology and immunohistochemistry
Native or HOPE-fixed vitrectomy specimens were rapidly transported to the Institute of Pathology and immediately processed. Cytospins were prepared by centrifugation at 1100 rpm for 4 minutes. Cytologic evaluation was performed with May-Grünwald-Giemsa stains. Immunocytochemical stains for CD20 and CD3 (Dako, Glostrup, Denmark, 1:250) were performed on an automated immunostainer (Ventana Medical Systems, Tucson, AZ) after 30 minutes of paraformaldehyde fixation. Additional stains were added depending on initial findings and available material.
DNA isolation and clonality analysis of IGH rearrangements
DNA was extracted from the remaining vitrectomy material according to previously published standard procedures.20 After assessment of DNA quality by control polymerase chain reaction (PCR) using the BIOMED-2 ladder, PCR for the detection of IGH gene rearrangements was performed in duplicate as previously described using BIOMED-2 FR2 and FR3 primers, sometimes complemented by additional analyses with BIOMED-2 IGH FR1 and κ primers (supplemental Methods, available on the Blood Web site).20
MYD88 mutation analysis
Detection of the MYD88L265P (c.728G>A) mutation was performed by melting curve analysis of amplification products with and without locked nucleic acid (LNA) for wild-type (WT) suppression, using the Light Cycler 480 II System (Roche Applied Science, Mannheim, Germany), as recently described.21 All VRL cases with MYD88L265 WT and selected VRL with MYD88L265P were subjected to Sanger sequencing of MYD88 exons 3 and 4 (supplemental Methods). The study was approved by the local ethics committee (105/2013BO2).
Results and discussion
Details of VRL patients are listed in Table 1. The test cohort included 31 males and 38 females (median age 73 years, range 23-94) who had undergone diagnostic vitrectomy for a suspected diagnosis of VRL. Based on cytologic findings and IGH PCR results, a diagnosis of VRL was made in 14 of 69 patients (20%). Clonal IGH rearrangements were found in 12 of 14 patients. In the remaining 2 cases (4 and 11) with negative or inadequate clonality results, VRL diagnosis was based on increased numbers of large atypical B cells (Figure 1B, top). A diagnosis of inflammation or insufficient evidence for VRL was made in 55 patients (80%), including 5 cases with clonal IGH rearrangements, which showed low cellularity and/or a predominance of small lymphocytes (Figure 1B, bottom), and were considered suspicious but not diagnostic for VRL.
Melting curve analysis detected the MYD88L265P mutation in 13 of 69 patients (18.8%), including 7 of 14 patients (50%) with a diagnosis of VRL, and 6 of 55 patients (10.9%) diagnosed as negative (3 cases) or suspicious (3 cases) (Figure 1C). Sequencing confirmed the presence of MYD88L265P in all positive-tested cases and additionally identified a p.P258L in a MYD88L265 WT VRL sample. Review of clinical data and follow-up confirmed a diagnosis of VRL in 21 of 69 (30%) patients, including 13 of 14 with an initial VRL diagnosis and all 6 MYD88L265P patients originally considered negative or suspicious for VRL. MYD88 analysis increased the sensitivity of vitreous fluid analysis from 62% to 90.5% and the negative predictive value from 85.5% to 96%, without change in specificity. For only 2 patients with MYD88WT VRL and suspicious vitrectomy results and B-cell clonality, chorioretinal biopsy was required to confirm the diagnosis. A single MYD88WT patient, a 23-year-old HIV+ man, initially diagnosed as VRL based on numerous atypical cells and B-cell clonality, was reclassified as having viral retinitis, demonstrating the difficult differential diagnosis between viral retinitis and VRL.22
In the validation cohort, MYD88L265P was identified in 6 of 8 VRL cases (75%) and 0 of 13 reactive samples. In total, MYD88 mutations were identified in 20 of 29 (69%) VRL, with the canonical L265P in 19 of 20 patients. There was no significant association of MYD88 mutation status with age, sex, CNS involvement, or other clinicopathologic parameters. However, 2 mutated cases with secondary involvement by systemic DLBCL had a history of MYD88L265P-positive testicular lymphoma, another immune-privileged site with dominance of DLBCL of ABC type, common MYD88 mutations, and increased risk for CNS relapse.23-25 Case 20 represented intraocular involvement by marginal zone B-cell lymphoma, which is usually negative for MYD88 mutations.
The high frequency of MYD88 mutations in VRL observed in this large series mirrors published data on PCNSL and provides further evidence that primary VRL and PCNSL represent the same entity. Most importantly, mutational analysis, especially detection of p.L265P with an allele-specific PCR technique,21 significantly increases the sensitivity of vitreous body analysis (Table 1). Poor cytologic preservation, low cellularity, and spurious occurrence of clonal or pseudoclonal PCR result in an unsatisfactory high frequency of false-negative and occasional false-positive results in vitreous body aspirates, often leading to significant delays in diagnosis and commencement of therapy.8,9 The presence of MYD88L265P in the majority of VRL allows a definitive lymphoma diagnosis even in poor quality samples, representing a significant diagnostic advance in this difficult and rare entity. Detection of MYD88L265P will enable the more timely commencement of treatment in mutation-positive cases, and might be used for monitoring treatment response (eg, using small-volume aqueous taps), thus potentially contributing to an improved outcome in VRL/PCNSL patients.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the contributing clinicians for their support and S. Colak and U. Beck-Bernhard for their technical assistance.
Authorship
Contribution: I.B. and F.F. designed the study; I.B., S.G., and J.S. performed the experiments; C.D., M.Z., M.W., P.S., D.S., B.F., S.E.C., and K.U.B.-S. contributed samples, evaluated data, and performed clinicopathologic correlations; I.B., S.E.C., L.Q.-M., K.U.B.-S., and F.F. interpreted the results; I.B. and F.F. wrote the manuscript; and all authors critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Falko Fend, Institute of Pathology and Neuropathology, University Hospital Tübingen, Liebermeisterstrasse 8, 72076 Tübingen, Germany; e-mail: falko.fend@med.uni-tuebingen.de.