Key Points
CNS involvement at relapse/progression in PTCL occurred at a frequency similar to what is seen in aggressive B-cell lymphomas.
Outcome after relapse is generally very poor in patients with PTCL and is not significantly altered by presence of CNS involvement at relapse.
Abstract
Central nervous system (CNS) relapse in non-Hodgkin lymphoma (NHL) carries a very poor prognosis. Risk factors and outcome have been studied in aggressive B-cell lymphomas, but very little is known about the risk in peripheral T-cell lymphoma (PTCL). We aimed at analyzing risk factors for CNS involvement at first relapse or progression, as well as the outcome of these patients, in a large population-based cohort of patients with PTCL. Twenty-eight out of 625 patients (4.5%) developed CNS disease over time. In multivariable analysis, disease characteristics at diagnosis independently associated with an increased risk for later CNS involvement were involvement of more than 1 extranodal site (hazard ratio [HR], 2.60; 95% confidence interval [CI], 1.07-6.29; P = .035) and skin (HR, 3.51; 95% CI, 1.26-9.74; P = .016) and gastrointestinal involvement (HR, 3.06; 95% CI, 1.30-7.18; P = .010). The outcome of relapsed/refractory patients was very poor, and CNS involvement was not associated with a significantly worse outcome compared with relapsed/refractory patients without CNS involvement in multivariable analysis (HR, 1.6; 95% CI, 0.96-2.6; P = .074). The results from the present study indicate that CNS relapse in PTCL occurs at a frequency similar to what is seen in aggressive B-cell lymphomas, but the poor outcomes in relapse are largely driven by systemic rather than CNS disease.
Introduction
In patients with aggressive non-Hodgkin lymphoma (NHL), disease relapse involving the central nervous system (CNS) has a dismal prognosis.1 It is an uncommon event, with incidence numbers ranging from 2% to 7% in large cohorts dominated by aggressive B-cell lymphomas.2-9 Risk factors for CNS relapse have not been entirely consistent between studies but have included specific anatomical sites, high proliferation rate, and advanced disease.2-4,8,10 The addition of intrathecal treatment to chemotherapy has commonly been used to avoid CNS relapse in patients deemed at high risk. However, there is limited evidence for this approach in patients treated with chemotherapy only,11,12 and in diffuse large B-cell lymphoma treated with rituximab in addition to chemotherapy, no beneficial effect of intrathecal treatment has been demonstrated.5,7,13 Similarly, the addition of etoposide to CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) treatment has been associated with a lower incidence of CNS relapse, an association lost when rituximab is added.3,5
In peripheral T-cell lymphoma (PTCL), CNS relapse has been little studied. A single-center review reported CNS relapse in 6 (2.4%) of 250 patients, and in a population-based study on relapsed PTCL, 12 (7.8%) of 153 relapses included the CNS.14,15 The most extensive study in PTCL included 228 patients, with extranodal NK/T-cell lymphoma excluded, and found an incidence of 8.8% CNS relapses.16 In that study, the identified risk factors for CNS relapse were elevated lactate dehydrogenase and paranasal sinus involvement. Extranodal NK/T-cell lymphoma, a disease that often involves the paranasal sinuses, was reported separately, with an incidence of 5.8% among 208 patients with this particular lymphoma.17
Consistent with studies in aggressive B-cell lymphomas, a poor survival was reported for patients with PTCL with CNS relapse,16 but several studies have also highlighted the dismal prognosis of relapsed PTCL in general.15,18,19
In this study, we aimed at analyzing risk factors for CNS involvement in first relapse and the outcome of these patients in a large population-based cohort of patients with PTCL.
Patients and methods
Patients
From the Swedish Lymphoma Registry (SLR), all patients diagnosed with T-cell lymphomas between January 1, 2000 and December 31, 2009 were identified. Compared with the compulsory Swedish Cancer Registry, the SLR covers approximately 95% of all adult (age, ≥18 years) lymphoma patients in Sweden, with further details described previously.20 The diagnosis of PTCL was established in routine clinical care with contributions from 21 pathology centers. At the time of diagnosis, approximately 75% of all cases were reviewed by expert hematopathologists at 7 large academic centers. There was no separate pathology review performed in the study. Each pathology report was retrospectively reviewed to avoid incorrect registry entries and for classification according to the 2008 edition of the World Health Organization classification of lymphoid neoplasms.21 Cases not fulfilling criteria for T-cell lymphoma or fulfilling criteria for precursor T-cell malignancies, primary cutaneous lymphomas, and leukemic forms were excluded from the study. Patients with CNS involvement at diagnosis were excluded from this study with the caveat that, in lack of neurologic symptoms, diagnostic evaluation of cerebrospinal fluid and CNS imaging were not routinely assessed at diagnosis as part of the standard clinical workup of these patients. Data were collected from the SLR, and further data on relapse was derived from a review of patient records, which for living patients required informed consent. The study was approved by the Regional Ethical Board, Lund, Sweden, and conducted in accordance with the Declaration of Helsinki.
Diagnosis of CNS disease
CNS relapse was defined as CNS involvement at any time after initial diagnosis and classified as isolated if no evidence of lymphoma relapse or progression outside the CNS was present. If the latter was present, it was classified as part of a systemic relapse. CNS disease was defined by clinical symptoms in association with either a radiological or cytological finding. Radiological findings by magnetic resonance imaging or computed tomography suggestive of lymphoma involvement of the brain was classified as parenchymal disease, whereas radiological findings with contrast enhancement of the meninges was classified as leptomeningeal disease. Clinical symptoms with cytological findings of malignant cells or atypical lymphocytes in cerebrospinal fluid, without any other reasonable explanation than lymphoma, was classified as leptomeningeal disease.
Statistical analysis
Time to CNS relapse was defined as time from diagnosis to diagnostic procedure demonstrating CNS involvement. Overall survival after first relapse was defined as time from relapse or progression to death from any cause or latest follow-up. Treatment response was classified according to tumor size change, as delineated in the international harmonization criteria,22 although measurements were made in routine care and not carried out as rigorously as in a clinical trial. Small residual pathological masses were classified as partial response in the absence of positron emission tomography-computed tomography evaluation. Response was in most cases assessed by review of computed tomography scan reports, or if missing or unavailable, response was assessed from physician notes. Distribution differences between groups were analyzed with χ-square test or Fisher’s exact test. Survival curves were estimated with the Kaplan-Meier method. Groups were compared using log-rank test, and risk factor analysis was made by Cox proportional hazards regression. Factors were analyzed in univariable analysis and factors with P ≤ .1 were analyzed using stepwise forward selection to create a multivariable model. All P-values were 2-sided, and values were regarded statistically significant if P < .05. All statistics were performed with IBM SPSS version 22.0 (SPSS Inc., Chicago, IL).
Results
Patient characteristics
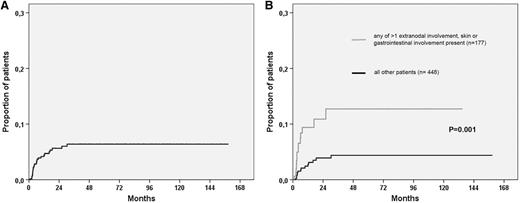
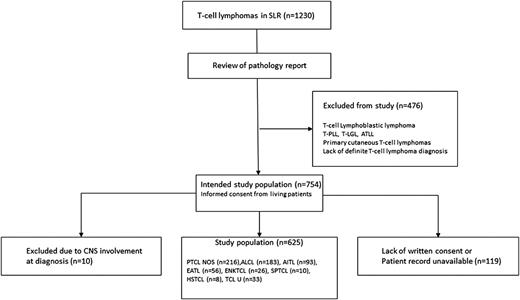
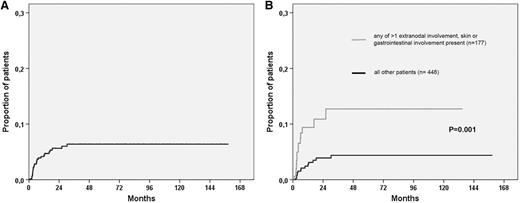
A total of 754 patients with PTCL were identified through the SLR, as illustrated in Figure 1. Ten of these patients had CNS involvement at diagnosis and were excluded from further analyses. For the remaining 744 patients, detailed data were available for 625 (84%) patients, and among these patients, 369 experienced relapse or progression. Clinical characteristics for the 625 patients according to CNS relapse status are listed in Table 1. Twenty-eight (4.5%) of 625 patients experienced a progression or relapse that involved the CNS, with a median time to CNS event of 4.3 months (range, 1.1-30 months). The Kaplan-Meier estimate for time to CNS relapse is shown in Figure 2A, with an actuarial incidence of 5.5% at 2 years, after which there were only 2 events and the curve seemed to reach a plateau. Twelve patients relapsed after responding to primary therapy (7 complete response, 5 partial response), and 15 nonresponding patients experienced CNS progression (1 stable disease [SD] SD, 14 progressive disease [PD]), whereas 1 patient was not evaluable with regard to response.
Patient cohort selection. AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; EATL, enteropathy-associated T-cell lymphoma; ENKTCL, extranodal NK/T-cell lymphoma, nasal type; HSTCL, hepatosplenic T-cell lymphoma; PTCL NOS, peripheral T-cell lymphoma not otherwise specified; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; TCL U, T-cell lymphoma unspecified; T-LGL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia.
Patient cohort selection. AITL, angioimmunoblastic T-cell lymphoma; ALCL, anaplastic large cell lymphoma; ATLL, adult T-cell leukemia/lymphoma; EATL, enteropathy-associated T-cell lymphoma; ENKTCL, extranodal NK/T-cell lymphoma, nasal type; HSTCL, hepatosplenic T-cell lymphoma; PTCL NOS, peripheral T-cell lymphoma not otherwise specified; SPTCL, subcutaneous panniculitis-like T-cell lymphoma; TCL U, T-cell lymphoma unspecified; T-LGL, T-cell large granular lymphocytic leukemia; T-PLL, T-cell prolymphocytic leukemia.
Cumulative risk for CNS relapse. Kaplan-Meier estimates of CNS relapse in (A) all patients. (B) Risk according to the presence of at least 1 versus none of the risk factors identified in multivariable analysis (>1 extranodal involvement, skin involvement, and gastrointestinal involvement).
Cumulative risk for CNS relapse. Kaplan-Meier estimates of CNS relapse in (A) all patients. (B) Risk according to the presence of at least 1 versus none of the risk factors identified in multivariable analysis (>1 extranodal involvement, skin involvement, and gastrointestinal involvement).
Pattern of CNS relapse
Relapse pattern is displayed in Table 2. Leptomeningeal involvement was more frequent than parenchymal relapse and was seen in 9 of 12 patients with response to primary treatment compared with 8 of 15 nonresponders, a difference that is not statistically significant (P = .4). Diagnostic procedures included magnetic resonance imaging in 16 patients, with computed tomography being used in the remaining patients. With the exception of 6 patients with parenchymal lesions on radiologic imaging, cerebrospinal fluid analysis was carried out in all patients and included flow cytometry in addition to microscopy in 10 of the cases. In our series, all cases with radiological findings in the leptomeninges also had cytological findings consistent with lymphoma involvement in the cerebrospinal fluid.
Treatment
All patients with established CNS relapse had received chemotherapy with curative intent; ie, CHOP (n = 17), CHOEP (CHOP plus etoposide) (n = 10), and CHOP plus alemtuzumab (n = 1), and 5 of the patients were treated with up-front autologous stem cell transplantation. Two of the CNS relapsed patients received prophylactic intrathecal methotrexate, and 1 received liposomal cytarabine. First-line treatment of the entire cohort is shown in Table 1, and the majority of the patients (75%) received CHOP or CHOEP, whereas 6% received other combination chemotherapy with curative intent. Fifty-one patients (8%) received intrathecal treatment as part of the primary treatment, and this proportion was 9% for patients treated with CHOP-like therapy.
Risk factors for CNS relapse
Clinical characteristics in Table 1 and all specific extranodal anatomic localizations recorded in patients with CNS relapse were tested in univariable analysis. Factors with P ≤ .1 for CNS relapse in univariable Cox proportional hazards regression and tested in the multivariable model are listed in Table 3. In the whole cohort, more than 1 extranodal involvement and skin and gastrointestinal involvement were independent risk factors for CNS relapse. The Kaplan-Meier estimates for CNS relapse in patients with at least 1 compared with none of the risk factors are shown in Figure 2B, with a HR of 3.2 (95% CI, 1.52-6.69; P = .002) for CNS involvement if any risk factor was present. The same risk factor analyses as described earlier were performed, selecting patients treated with CHOP (n = 320) or CHOEP (n = 150), and for CNS involvement only in first relapse or progression, resulting in the factors displayed in Table 3 retaining their significance in multivariable analysis (data not shown).
Among the CHOP/CHOEP-treated patients, we also analyzed the possible protective effect of treatment modifications. Intrathecal injections and addition of etoposide did not show any association with a reduced risk for CNS relapse in univariable analysis (HR, 1.3 [P = .7] and HR, 1.1 [P = .8], respectively); similarly, no association was found with up-front autologous stem cell transplantation (HR, 0.7; P = .4).
Outcome
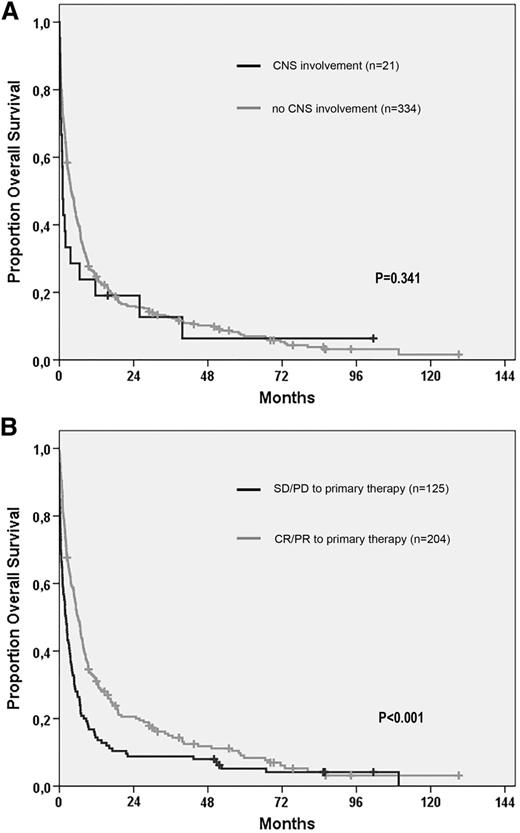
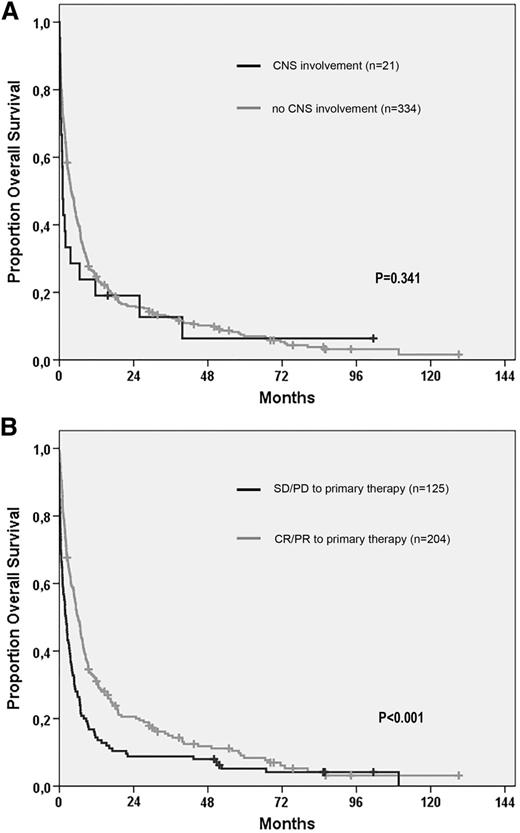
Median overall survival from relapse or progression was 1.1 months for all patients with CNS involvement and 3.8 months for patients without CNS involvement (log-rank P = .082). Excluding the patients with CNS involvement in second or later relapse (n = 7), survival from first relapse, or progression in relation to CNS involvement is illustrated in Figure 3A. At first relapse or progression, there was no difference in the proportion of patients who received treatment according to CNS involvement or not (67% for both groups), and CNS disease was not associated with a shorter survival (HR, 1.25 [(5% CI, 0.79-1.99]; P = .345) in univariable Cox proportional hazards regression analysis. Factors that predicted a shorter survival from first relapse or progression in multivariable analysis were age at relapse (HR, 1.025 per year; 95% CI, 1.016-1.034; P < .001), male gender (HR, 1.3; 95% CI, 1.1-1.7; P = .017), and disease not responding (SD/PD) to primary treatment (HR, 1.6; 95% CI, 1.3-2.1; P < .001), with survival according to the latter factor illustrated in Figure 3B. When CNS involvement was incorporated in the multivariable model, it was still not associated with a significantly worse outcome (HR, 1.6; 95% CI, 0.96-2.6; P = .074). Detailed information on factors such as World Health Organization performance status at relapse/progression was not available.
Overall survival from time of relapse in patients with PTCL. (A) Survival according to CNS involvement or not at first relapse. (B) Survival of relapsed patients according to response (complete response or partial response) or refractoriness (SD or PD) to primary treatment.
Overall survival from time of relapse in patients with PTCL. (A) Survival according to CNS involvement or not at first relapse. (B) Survival of relapsed patients according to response (complete response or partial response) or refractoriness (SD or PD) to primary treatment.
Discussion
This is one of very few studies on CNS relapse in PTCL, and the largest population-based study to address this question. In our study, 4.5% of the patients developed CNS involvement at relapse or progression, a proportion very similar to several studies in aggressive NHL, dominated by B-cell lymphomas, in which an incidence of approximately 5% is reported.2-4 The incidence of CNS relapse in our study falls in between the 2 previous studies focusing on CNS relapse in PTCL, with the study by Yi et al16 reporting an incidence of almost 9%, whereas Pro et al reported a frequency of 2.6%.14 In a population-based study by Mak et al,15 12 (7.8%) of 153 relapsed patients with PTCL experienced CNS involvement. The proportion in our cohort in which 28 (7.6%) of 369 relapsed patients developed CNS disease is very similar and is consistent with the study by Mak et al, in which the majority of CNS involvements were found at first relapse or progression. It is of note that, apart from the 10 patients with CNS involvement at diagnosis, CNS relapse/progression was only diagnosed in patients treated with a curative intent in our cohort. A substantial proportion of patients in our series received palliative treatment or no treatment at all. The lack of CNS involvement among those patients could possibly be a result of the little value perceived by the treating physician to establish a CNS progression in a palliative setting. If only patients who received curative treatment (n = 505) are considered eligible for the diagnosis, the CNS relapse frequency would rise to 5.5% in our cohort. The great majority of CNS relapses in our series were seen within 2 years from diagnosis, and late CNS involvement seems to be very uncommon, even though we cannot exclude its occurrence.
Multivariable risk factor analysis revealed more than 1 site of extranodal involvement and gastrointestinal and skin involvement to be independent prognostic factors for CNS relapse in our cohort. Bone marrow involvement, international prognostic index, and elevated serum lactate dehydrogenase were not associated with CNS relapse. The latter, together with paranasal sinus involvement, were the risk factors identified by Yi et al,16 but we could not confirm this association. Given that elevated lactate dehydrogenase was a common feature in our cohort (58% of patients) and in other large series of PTCL,23,24 we believe this factor might be of limited importance as a predictor for CNS relapse in PTCL. Very few patients had involvement of some particular organs, such as testicles or kidneys, which has been associated with a high incidence of CNS relapse in aggressive NHL,8,25 and the low number of patients in combination with the rarity of CNS involvement becomes a major limitation of our study in this aspect. There were no apparent differences in the frequency of CNS relapse among the more common subtypes in our data, supporting the hypothesis that CNS involvement may be more uncommon in angioimmunoblastic T-cell lymphoma suggested by Yi et al.16 The low number of patients and the lack of central pathology review, however, limits conclusions to be drawn from our study. On the basis of our findings, the incorporation of magnetic resonance imaging and cerebrospinal fluid analysis as part of initial work-up for high-risk patients, for example, patients with extensive extranodal involvement, might be considered. This should, however, be evaluated in a prospective study before it could be generally recommended.
Apart from a few patients included in studies in aggressive NHL, the role of CNS prophylaxis has never been studied in PTCL. Given the low incidence of PTCL, this will probably remain a question without a definite answer. On the basis of our results, it seems that, similar to aggressive B-cell lymphomas, there is a subset of patients with PTCL who could potentially benefit from CNS prophylaxis. Comparison between B-cell and T-cell malignancies is not necessarily relevant, as disease characteristics and biology are markedly different, but available studies on CNS prophylaxis are dominated by aggressive B-cell lymphomas. The evidence for a beneficial effect of CNS prophylactic treatment is conflicting,3,5,11,12,26 and whether intrathecal prophylaxis or systemic treatment, for example, intravenous high-dose methotrexate, would be preferred in patients with PTCL is unclear. Our study shows that both parenchymal and leptomeningeal disease occur, but we found no association of intrathecal injections or the addition of etoposide with a lower risk for CNS relapse. With regard to treatment modifications, the retrospective nature of our study is an important limitation, and at least the former treatment modification has probably been made in patients judged to have a high risk for CNS relapse, hence introducing a substantial bias.
CNS relapse is known to have a very poor prognosis from several studies in aggressive NHL.2-5 Despite the dismal outcome of patients with CNS disease at relapse in our study, this did not translate into a significantly worse prognosis compared with patients with relapses outside the CNS. In contrast to Yi et al, we performed the survival analysis from time of relapse, thereby more clearly illustrating the general poor outcome of patients with PTCL at disease progression or relapse. Because CNS involvement is such an infrequent complication, and is often found with concurrent systemic disease progression, one might argue that at present an optimal CNS prophylaxis is of less concern compared with developing better treatment of systemic disease both up-front and at relapse.
In conclusion, CNS involvement at relapse or progression in a large population-based cohort of PTCL, appeared at a similar frequency as in aggressive B-cell NHL. Extensive extranodal manifestations, particularly with involvement of skin and the gastrointestinal tract, identified patients with a higher risk for CNS disease at relapse. Patients with CNS involvement had a very poor survival, but that was true also for all relapsed patients. Taking into account the rarity of CNS spread, the poor outcomes in relapsed patients with PTCL is largely driven by the lack of effective salvage treatment of systemic disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all clinicians contributing to the Swedish Lymphoma Registry. We also thank Karin Ekström Smedby, Hans Hagberg, Lena Hermansson, Christina Holm, Daniel Molin, Niklas Theorin, and Lena Wigren, who were very helpful during data collection, and Lars Brudin for advice in statistical analyses.
This study was in part supported by an unrestricted grant from the Swedish Hematology Association in collaboration with Roche.
Authorship
Contribution: F.E., M.J., and T.R. designed the research; F.E. and J.L. collected data; F.E., M.J., and T.R. analyzed data; and F.E., M.J., J.L., and T.R. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fredrik Ellin, Department of Internal Medicine, Kalmar County Hospital, S-391 85 Kalmar, Sweden; e-mail: fredrik.ellin@med.lu.se.