In this issue of Blood, Schramm et al demonstrate that the majority of mutations in complement C3 identified in atypical hemolytic uremic syndrome (aHUS) patients cause dysregulation in the alternative pathway of complement.1
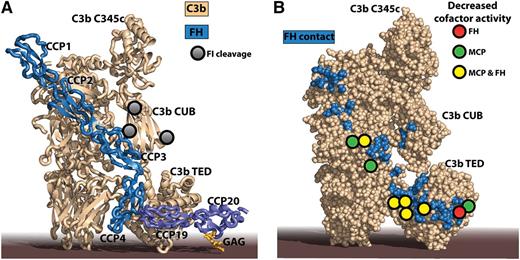
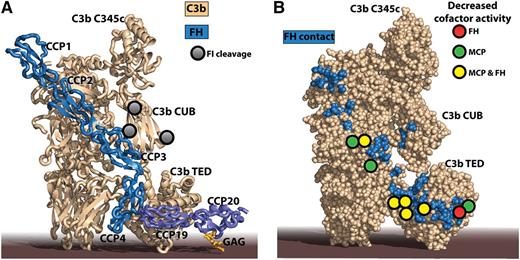
The impaired FI degradation of mutant C3 molecules identified in aHUS patients can be rationalized by crystal structures of C3b-FH complexes. (A) The structure of C3b in complex with FH CCP domains 1 to 46 combined with that of C3d in complex with FH CCP19-20 and a model GAG.7 C3d is a final degradation product of C3b, comprising basically only the thioester-containing domain (TED). Notice that the 2 extremes of FH may bind to the same C3b molecule with the intervening CCP domains looping out,8 but simultaneous interaction of FH with 2 activator-bound C3b molecules cannot be excluded either. FH CCP1-4 is recognized by 4 MG domains, the CUB domain, and the TED in C3b, whereas FH CCP19-20 is only contacting the C3b TED. An additional complication is that FH CCP19 interacts with the C3b TED, whereas FH CCP20 can interact with host cell membrane-linked GAG as indicated here or a nearby molecule of C3d.9 (B) A large number of mutant C3 residues identified in aHUS patients by Schramm et al give rise to lower cofactor activity of FH and MCP (colored circles). They are all located directly in or near to the binding interface for FH on C3b (blue residues). The figure was prepared with PyMOL10 by the combination of Protein Data Bank ID codes 4ONT7 and 2WII.6
The impaired FI degradation of mutant C3 molecules identified in aHUS patients can be rationalized by crystal structures of C3b-FH complexes. (A) The structure of C3b in complex with FH CCP domains 1 to 46 combined with that of C3d in complex with FH CCP19-20 and a model GAG.7 C3d is a final degradation product of C3b, comprising basically only the thioester-containing domain (TED). Notice that the 2 extremes of FH may bind to the same C3b molecule with the intervening CCP domains looping out,8 but simultaneous interaction of FH with 2 activator-bound C3b molecules cannot be excluded either. FH CCP1-4 is recognized by 4 MG domains, the CUB domain, and the TED in C3b, whereas FH CCP19-20 is only contacting the C3b TED. An additional complication is that FH CCP19 interacts with the C3b TED, whereas FH CCP20 can interact with host cell membrane-linked GAG as indicated here or a nearby molecule of C3d.9 (B) A large number of mutant C3 residues identified in aHUS patients by Schramm et al give rise to lower cofactor activity of FH and MCP (colored circles). They are all located directly in or near to the binding interface for FH on C3b (blue residues). The figure was prepared with PyMOL10 by the combination of Protein Data Bank ID codes 4ONT7 and 2WII.6
The underlying molecular mechanism is shown to be decreased cofactor activity of complement regulators. The resulting increased formation and stability of the C3 degradation product C3b are translated into increased C3 deposition onto endothelial cells, and the majority of aHUS patients carrying mutations in C3 were observed to exhibit low levels of plasma C3. The mechanistic outcome of the study is the very similar effects observed for the majority of the C3 mutations on the cofactor activity of factor H (FH) and membrane cofactor protein (MCP), suggesting that their binding surfaces on C3b are strongly overlapping.
C3b is generated by proteolysis of C3 on complement activation. It recruits factor B (FB), which is subsequently cleaved into Bb, and thus the alternative pathway (AP) C3 convertase C3bBb is formed, and a positive feedback amplification loop leads to further convertase assembly. This amplification is suppressed on healthy host cells by complement regulators.2 One of the best studied complement regulators is FH, which binds to host surfaces through sialic acid, heparin, and sulfated glycosaminoglycans (GAGs) and in this manner attenuates the AP on host cells. FH accelerates convertase decay by irreversibly dissociating Bb from the C3 convertase and may subsequently serve as cofactor for the serine protease factor I (FI), which cleaves C3b into iC3b, which is unable to rebind FB. Two other cofactors are MCP and complement receptor 1 (CR1), which in contrast to the soluble FH are anchored in host cell membranes.2
The pathogenesis of aHUS leading to endothelial damage and microvascular thrombosis has long been linked to impaired complement regulation.3 Genetic defects leading to mutations in the components of the alternative pathway or FH autoantibodies have been identified in roughly 60% of aHUS patients. Mutations are commonly found in FH and MCP, whereas mutations within C3 and FI occur at lower frequencies.3 Patients with C3 mutations develop severe disease, leading to end-stage renal failure in 55% to 65% of cases. The terminal complement pathway is required for the induction of endothelial lesions in aHUS.4
Schramm et al constructed their database of mutations by sequencing the C3 gene in almost 1300 aHUS patients from France, Italy, the United Kingdom, and the United States. In combination with already published mutations, they could list 48 different genetic changes in C3 associated with aHUS. Using the C3b-containing crystal structure, the authors could hypothesize why the mutations conferred AP dysregulation. A striking finding was that 27 of the mutated residues map within or close to the binding sites of FH CCP domains 1 to 4 (CCP1-4) or CCP19-20 on C3b (see figure panel A), suggesting that these mutations affect the cofactor activity of FH, leading to decreased conversion of C3b to iC3b. A similar explanation could be offered for a single mutant residue close to a FI cleavage site in the complement C1r/C1s, Uegf, Bmp1 (CUB) domain of C3b. To experimentally investigate how the C3 mutants induce dysregulation of the AP, the authors prepared an impressive collection of 23 mutant recombinant C3 molecules. One caveat concerning this collection is that, due to the procedure for recombinant expression, these C3 variants do not contain the internal thioester found in circulating C3. Instead the recombinant proteins were in the form of the naturally occurring thioester tick-over product C3(H2O). This adopts a C3b-like conformation and is capable of forming a fluid phase AP C3 convertase with FB, and C3(H2O) is also subject to FI degradation.5 Hence, although the recombinant C3 variants still contain the anaphylatoxin domain released as C3a from C3 by C3 convertases, they represent a valuable model system for examining how mutations in C3 affect binding of cofactors and the subsequent conversion of C3b to iC3b by FI. However, it should be kept in mind that this model system only approximates the degradation of activator surface bound C3b to iC3b.
The recombinant C3 variants were evaluated for their binding to the immobilized cofactors FH, MCP, and CR1 through surface plasmon resonance. Of the 23 mutants evaluated, 17 displayed decreased binding to ≥1 of the 3 regulators compared with wild-type (WT) C3. Intriguingly, the authors observed a very strong correlation between the binding of the C3 variants to FH and MCP, suggesting that their C3 binding modes are very similar. In contrast, binding to CR1 was much less affected. The impact of the mutations was further investigated by examining cofactor activity when mutant C3 acted as a substrate for FI. For 14 C3 variants examined, 12 showed diminished cofactor activity for MCP or FH compared with degradation of WT C3, whereas both regulators displayed a lowered cofactor activity against 8 C3 variants (see figure panel B). The C3 mutations associated with lower cofactor activity were located in or around the binding sites for FH CCP2-4 and CCP19-20.6-9 In contrast, CR1 cofactor activity was much less affected by mutations in C3.
An impaired degradation of C3b to iC3b predicts an increased C3 deposition on self-tissue from patients. To validate this, Schramm et al measured the deposition of C3 onto human umbilical vein endothelial cells (HUVECs) by FH-depleted serum. One patient serum contained C3 with the A1072D mutation, which in the FI degradation assay gave rise to a lower cofactor activity of both FH and MCP, whereas another patient serum had C3 with the mutation R139W, which only gave lower cofactor activity for MCP. As predicted, with serum containing C3 A1072D, much more exogenous FH was required to decrease C3 deposition on HUVECs compared with serum containing either C3 R139W or WT C3.
The work of Schramm et al further strengthens the idea that the mechanisms keeping the complement AP in check on host cells are compromised in aHUS patients. The authors also demonstrate in an elegant manner how the combination of genetic data from a large number of patients, functional assays, and structural biology paves the way for personalized medicine.
Conflict-of-interest disclosure: The author declares no competing financial interests.