In this issue of Blood, Airoldi et al find that γδ T cells can provide critical antiviral and antileukemia effects after HLA-haploidentical hematopoietic transplants depleted of αβ T cells.1
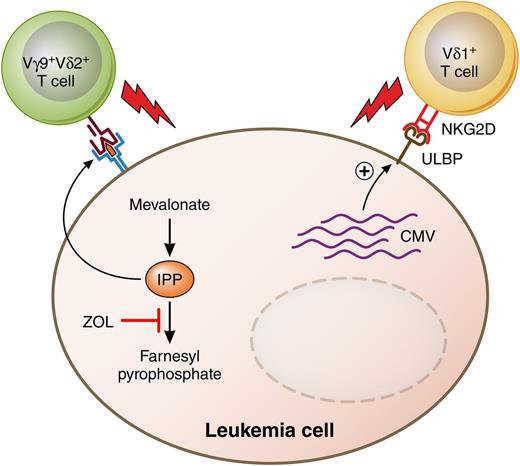
Putative antileukemia anti-CMV activities of human γδ TCR-bearing T cells. ZOL and other bisphosphonates inhibit a key enzyme of the mevalonate pathway of cholesterol biosynthesis, leading to accumulation of IPP in acute leukemia cells. IPP is one of the nonpeptide phosphoantigens that are recognized by and stimulate cytolysis by human Vγ9+Vδ2+ T cells. Meanwhile, human CMV glycoprotein UL16 binds to the MHC class I–related molecule UL16-binding protein (ULBP) and stimulates the natural killer cell stimulatory receptor NKG2D, also found on Vδ1+ T cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
Putative antileukemia anti-CMV activities of human γδ TCR-bearing T cells. ZOL and other bisphosphonates inhibit a key enzyme of the mevalonate pathway of cholesterol biosynthesis, leading to accumulation of IPP in acute leukemia cells. IPP is one of the nonpeptide phosphoantigens that are recognized by and stimulate cytolysis by human Vγ9+Vδ2+ T cells. Meanwhile, human CMV glycoprotein UL16 binds to the MHC class I–related molecule UL16-binding protein (ULBP) and stimulates the natural killer cell stimulatory receptor NKG2D, also found on Vδ1+ T cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
HLA-haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is emerging as a safe and effective treatment option for patients with hematologic malignancies who lack an HLA-matched sibling or unrelated donor. It was not always this way. Early studies of haplo-HSCT using T-cell–replete bone marrow grafts and conventional, cyclosporine-based graft-versus-host disease (GVHD) prophylaxis reported high incidences of hyperacute GVHD, acute GVHD, and nonrelapse mortality, as well as poor overall and event-free survival.2 A major breakthrough in haplo-HSCT came through the development of graft engineering methods, first by soybean agglutination and E-rosetting, and then by magnetic cell selection technologies. The problem of GVHD after haplo-HSCT was solved by transplanting high doses of CD34+ cells obtained by positive selection3 ; however, this approach created 2 new potential problems: (1) poor immune reconstitution with increased infectious mortality and (2) an increased risk of relapse due to the depletion of conventional T cells and the loss of a T-cell–mediated graft-versus-leukemia effect.
Different approaches have been taken to address immune reconstitution and relapse after T-cell–depleted haplo-HSCT. One approach is to infuse CD4+CD25+ regulatory T cells to prevent GVHD by a subsequent infusion of conventional CD4+CD25− T cells.4 Another approach is to use negative selection techniques to deplete only the cells that cause GVHD while preserving cells of the innate immune system such as monocytes, macrophages, dendritic cells, and natural killer cells. The first generation of negative selection techniques used depletion of CD3+ T cells to reduce the risk of GVHD, and CD19+ B cells to reduce the incidence of Epstein-Barr virus–induced lymphoproliferative disease. More recently, depletion of CD3+ T cells has been replaced by removal of only those T cells expressing the conventional αβ T-cell receptor (TCR-αβ), leaving the population of T cells expressing the γδ receptor (TCR-γδ) in the graft. TCR-γδ+ T cells are a distinct population of adaptive lymphocytes whose functions are distinct from those of conventional TCR-αβ T cells.5 TCR-γδ+ T cells comprise lymphoid homing γδ T cells that clonally expand in an “adaptive” manner as well as innate-like cells that home to tissues such as epidermis, dermis, intestine, lungs, and uterus. They are not restricted to recognize peptides bound to major histocompatibility complex (MHC) molecules, and the Vγ9+Vδ2+ subset can recognize metabolites of the mevalonate pathway on the surface of tumor cells6 and kill acute myeloid leukemia blasts7 (see figure). Finally, reactivation of human cytomegalovirus (CMV) after allogeneic stem cell transplantation is associated with the in vivo expansion of Vδ2− γδ T cells that react against CMV-infected cells, and such expansion is correlated with clearance of the virus.8
In this issue, Airoldi and colleagues characterize functional and phenotypic reconstitution of γδ T cells in 27 children with malignant or nonmalignant disorders treated with haplo-HSCT using grafts depleted of αβ T cells and CD19+ B cells.1 Compared with recipients of grafts positively selected for CD34+ cells, recipients of TCRαβ−/CD19− grafts had higher percentages of γδ T cells among total T cells and Vδ2+ cells among γδ T cells at 3 months after transplantation, demonstrating that selective retention of the γδ subset in the graft affects the kinetics and pattern of immune reconstitution following haplo-HSCT. Perhaps most relevant to clinicians, the reconstituting γδ T cells were found to have lytic activity against human cytomegalovirus and against acute myeloid leukemia blasts. The Vδ1+ subset expanded significantly in the 15 patients who experienced CMV reactivation and contained more terminally differentiated cells, suggesting that this subset was actually fighting the virus in vivo. Furthermore, ex vivo–expanded Vγ9+Vδ2+ T cells efficiently killed acute myeloid leukemia or acute lymphoid leukemia blasts that had been exposed to zoledronic acid (ZOL), an aminobisphosphonate that induces the upregulation of isopentenyl pyrophosphate (IPP), a ligand of Vγ9+Vδ2+T cells, by inhibiting a key enzyme of the mevalonate pathway.9 Taken together, the results suggest that selective depletion of TCR-αβ+ T cells from HLA-haploidentical peripheral blood grafts enhances the functional and phenotypic reconstitution of TCR-γδ+ T cells and offers the possibility of reducing the risk of leukemia relapse by treating recipients with aminobisphosphonate drugs. Additionally, the ability of γδ T cells to kill both CMV-infected and leukemic targets may account for the possible correlation between CMV reactivation and a decreased risk of leukemia relapse10 (see figure).
How will the results of this study impact allogeneic stem cell transplantation? It is perhaps too early to tell. Follow-up studies of haplo-HSCT with TCR-αβ/CD19 depletion will need to focus on the correlation of immune reconstitution with the incidence of CMV reactivation and leukemia relapse. The possibility of reducing relapse by treating recipients with ZOL is intriguing but remains speculative. What the study does accomplish is to demonstrate the potential to impact immune reconstitution and graft-versus-leukemia effects through intelligent graft engineering. As haplo-HSCT gains in acceptance, more studies like this one will be needed to define exactly which cells are needed in the graft and why, and which ones can be thrown away.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal