In this issue of Blood, Zappasodi et al show that heat-shock protein 105 (HSP105/HSPH1) is preferentially expressed in aggressive B-cell lymphomas, it modulates BCL-6 and c-Myc expression, and its inhibition reduces the tumor growth in vivo, suggesting a novel target for aggressive B-cell lymphoma.1
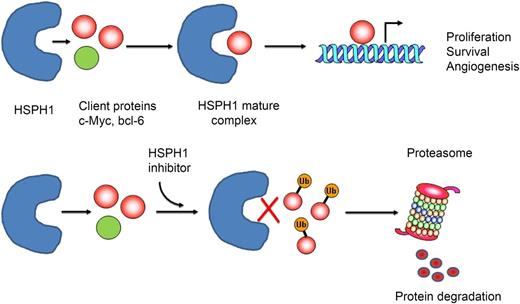
(Top) Heat-shock protein H1 (HSPH1) binds to client proteins (CPs) involved in lymphomagenesis (eg, c-Myc, BCL-6) to generate a “mature complex” that contributes to the stabilization and maturation of those proteins, promoting their cellular function. (Bottom) HSP inhibitors would prevent binding of CPs to HSPH1, remaining in the cytosol, where CPs are ubiquitinated (Ub) and subsequently degradated by the proteasome.
(Top) Heat-shock protein H1 (HSPH1) binds to client proteins (CPs) involved in lymphomagenesis (eg, c-Myc, BCL-6) to generate a “mature complex” that contributes to the stabilization and maturation of those proteins, promoting their cellular function. (Bottom) HSP inhibitors would prevent binding of CPs to HSPH1, remaining in the cytosol, where CPs are ubiquitinated (Ub) and subsequently degradated by the proteasome.
Heat-shock proteins (HSPs) are highly conserved proteins that act as molecular chaperones.2 They are involved in the regulation of folding, intracellular transportation, and proteolytic degradation of many proteins, playing a critical role in cell proliferation, signal transduction, apoptosis, and angiogenesis. In mammals, the HSP family consists of 5 members: HSP100, HSP90, HSP70, HSP60, and small HSPs including HSP27. Some proteins are constitutively expressed, whereas others are induced by cellular stress to protect and enhance cell survival. HSPs interact with a number of oncogenic proteins and receptors (eg, p53, c-Myc, Akt, cyclin D, Cdk4, ALK) that are deregulated in lymphoma, preventing their degradation (see figure). Overexpression of HSPs has been documented in different lymphoma subtypes and it contributes to the oncogenic process.3 Inhibition of HSPs that specifically bind to proteins involved in lymphomagenesis may be of great value for the treatment of these diseases.
The authors show that HSPH1, a subgroup of the HSP70 family of proteins, is expressed in B-cell lymphomas, preferentially in those with a high proliferation rate. HSPH1 protein expression directly correlates with BCL-6 and c-Myc protein expression in aggressive lymphoma cell lines. In fact, the authors convincingly show that HSPH1 directly binds to BCL-6 and c-Myc, thus acting as a chaperone for these proteins. Most important, in a series of in vitro studies, the authors show that HSPH1 inhibition directly downregulates BCL-6 and c-Myc, which leads to a significant decrease in cell proliferation. To further evaluate this concept, the group tested the growth of an aggressive B-cell lymphoma line in an in vivo model. Mice transplanted with HSPH1–knocked-down tumors had significantly longer survival, and the protein levels of BCL-6 and c-Myc in the tumor cells were decreased, in agreement with the reported in vitro studies. In addition, tumor angiogenesis was also reduced, consistent with the inhibition of the provascularization properties of c-Myc, which may additionally contribute to the antitumoral effect.
Finally, to enhance the clinical relevance of this finding, the investigators analyzed the expression of HSPH1 in primary B-cell lymphomas. In a previous study, they showed that HSPH1 protein expression was correlated with the tumor proliferation rate. Thus, low-grade lymphomas showed a significantly lower HSPH1 tumor expression than high-grade lymphomas.4 In particular, diffuse large B-cell lymphoma (DLBCL) with a high Ki-67 index and Burkitt lymphoma appear to have higher HSPH1 expression. In line with these data, HSPH1 protein expression in a transformed DLBCL was significantly increased compared with the expression seen in the indolent lymphoma. Remarkably, in c-Myc+ lymphomas, HSPH1 protein expression correlated with that of c-Myc.
Overall, these data suggest that HSPH1 inhibition could potentially be useful in those lymphomas with c-Myc alterations (ie, Burkitt and the so-called “double-hit” lymphomas—mature B-cell lymphomas with a translocation affecting c-Myc in combination with another translocation usually affecting bcl-2). This is of paramount relevance, in particular for the latter, amounting to a poor-prognosis lymphoma for which no effective therapies have been developed so far.5
A few studies have addressed the inhibition of other HSPs as a treatment of B-cell lymphoma. In particular, HSP90, an important chaperone involved in cancer,6 is frequently expressed in DLBCL and, importantly, its expression highly correlates with BCL-6,7 a transcription factor that is frequently deregulated in DLBCL. Experimental in vitro and in vivo studies showed that a purine-derived HSP90 inhibitor, PUH71, selectively killed primary DLBCL-coexpressing BCL-6 and HSP90, and this approach is being translated into the clinical scenario in patients with lymphoma with the new available HSP90 inhibitors. Although early trials with HSP inhibitors in lymphoma patients have not shown impressive clinical activity, it may be envisioned that a combination of different HSP inhibitors targeting distinct oncogenic proteins may improve their efficacy and may add value to conventional immunochemotherapy.
Pharmacologic inhibitors of HSPH1 have yet to be developed, but the discovery of HSPH1 as a chaperone of c-Myc in B-cell lymphoma presents an important opportunity to design rational, molecularly based treatments for c-Myc–dependent B-cell lymphomas such as Burkitt lymphoma and the “double-hit” DLBCL.
Conflict-of-interest disclosure: The author declares no competing financial interests.