Key Points
Remission status in relapsing-remitting AVWS depends on the balance of VWF clearance by anti-VWF antibody and VWF secretion.
VWFpp:Ag ratio is a simple assay that provides information on this balance and predicts remission status in this case of AVWS.
We investigated a case of acquired von Willebrand syndrome (AVWS) secondary to a nonneutralizing anti-von Willebrand factor (VWF) antibody associated with an autoimmune disorder. At diagnosis, VWF activity (VWF:Act), antigen (VWF:Ag), multimers, and factor VIII coagulant activity were virtually absent. VWF propeptide (VWFpp) was elevated with an infinitely high VWFpp to VWF:Ag ratio (VWFpp:Ag) consistent with rapid VWF clearance. Immunosuppressive treatment resulted in phenotypic remission 1 with normalization of VWF/factor VIII levels and multimer pattern. However, VWFpp:Ag remained elevated (∼2× normal), consistent with ongoing VWF clearance by the remaining anti-VWF antibody still present by enzyme-linked immunosorbent assay. This suggests that increased VWF secretion was compensating for the incomplete remission state. Relapse occurred when VWFpp:Ag was again infinitely high, with associated decreased VWFpp but unchanged anti-VWF titers; switching the balance to favor VWF clearance over secretion. Complete remission with undetectable anti-VWF occurred only when VWFpp:Ag was normal. This case of relapsing-remitting AVWS demonstrates the use of VWFpp:Ag for predicting remission status.
Introduction
Acquired von Willebrand syndrome (AVWS) is a rare bleeding disorder with clinical and laboratory features similar to congenital von Willebrand’s disease (VWD),1,2 but without a family or personal history of previous bleeding tendency.3 AVWS can be associated with various underlying disorders, including lymphoproliferative disorders, autoimmune disorders, and monoclonal gammopathies,4,-6 in which the common mechanism is inhibition or clearance of von Willebrand factor (VWF) by paraprotein or autoantibody.7
We report a case of AVWS resulting from a nonneutralizing IgG-autoantibody, resulting in the virtual absence of VWF and factor VIII (FVIII) levels. By following the relative titers of IgG anti-VWF, VWF antigen (VWF:Ag), activity (VWF:Act), and propeptide (VWFpp) throughout the patient's relapsing-remitting course, we were able to show how her laboratory phenotypic expression is determined by the balance of VWF clearance and secretion and how the VWFpp to VWF:Ag ratio (VWFpp:Ag) helps predict remission status.
Case
A previously healthy 18-year-old woman with no prior bleeding diathesis presented with myalgias, muscle weakness, and polyarthritis. An autoimmune disorder consistent with polymyositis with systemic lupus erythematous overlap was diagnosed on the basis of elevated inflammatory markers, creatinine kinase, low complement levels, and positive antinuclear antibodies, myositis autoantibody (Mi-2), and anti-double-stranded DNA antibody. Her new bleeding symptoms included spontaneous bruising, metromenorrhagia, and prolonged oozing after recent tooth extraction and venipunctures; none required hemostatic management. Activated partial thromboplastin time was prolonged with absent lupus-type or FVIII inhibitor. VWF:Ag, VWF:Act, and FVIII coagulant activity (FVIII:C) levels were virtually absent, with absent VWF multimers (Table 1; Figure 1A). Family studies were negative for congenital VWD.
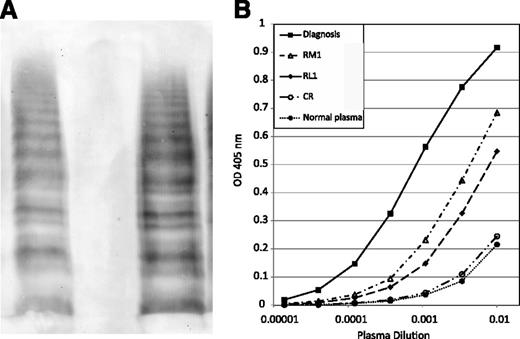
VWF multimer patterns and anti-VWF IgG titers. (A) Multimer analysis. Lane 1, normal control; lane 2, patient plasma at diagnosis; lane 3, patient plasma at RM1. All specimens were run on the same gel. (B) IgG anti-VWF antibody titer. All specimens were assayed in the same run for comparison.
VWF multimer patterns and anti-VWF IgG titers. (A) Multimer analysis. Lane 1, normal control; lane 2, patient plasma at diagnosis; lane 3, patient plasma at RM1. All specimens were run on the same gel. (B) IgG anti-VWF antibody titer. All specimens were assayed in the same run for comparison.
Treatment with prednisone and azathioprine resulted in prompt resolution of her symptoms and normalization of the patient's VWF/FVIII levels (Remission 1 [RM1]; Table 1). Several months later, her disease relapsed, with recurrence of arthralgias, elevated inflammatory markers, and dropping VWF/FVIII levels (Relapse 1 [RL1]; Table 1.) After a couple more remissions and relapses, she finally achieved complete remission (CR) when anti-VWF antibodies became undetectable.
Methods
Assay methods for FVIII:C, VWF:Ag, VWF:Act (latex-particle immunoturbidimetry for VWF-glycoprotein 1b domain), and VWF multimer analysis were as previously described.8 VWFpp was measured by VWFpp-specific monoclonal antibody enzyme-linked immunosorbent assay (ELISA), using the GTI Diagnostics kit. VWF antibody was determined by ELISA in 96-well plates. Fifty microliters heat-treated (56°C × 30 minutes) patient plasma (serially diluted in phosphate-buffered saline containing 1% bovine serum albumin/tween20/Thimersol, at pH 7.4) or normal plasma was added to wells coated with recombinant VWF (rVWF, kindly provided by Baxter Inc.). After incubation, washing, and blocking with 3% bovine serum albumin in phosphate-buffered saline/Thimersol at pH 7.4, biotin-conjugated goat anti-human immunoglobulin (IgG, IgA, or IgM) and Streptavidin-alkaline phosphatase were sequentially added, each followed by incubation and washing. Alkaline phosphatase substrate p-nitrophenyl phosphate was used for colorimetric quantification of bound patient anti-VWF at 405 nm optical density.
Results/discussion
In our patient, we demonstrate nonneutralizing antibody-mediated VWF clearance as the mechanism responsible for her AVWS (Table 1; Figure 1). Mixing studies (37°C × 2-hour incubation) showed no inhibitory effect on normal plasma VWF:Act and FVIII:C levels, consistent with antibody against nonfunctional VWF domains. Plasma VWF multimers were absent at diagnosis but present with normal pattern at RM1 and CR (Figure 1A). Anti-VWF antibody present at diagnosis was predominantly IgG, with minor IgM and no IgA (data not shown). Figure 1B shows that at RM1 IgG anti-VWF remained present at a lower titer, and IgM anti-VWF was absent. IgG anti-VWF titer at RL1 was similar to that at RM1, but then ultimately disappeared in CR.
VWFpp levels and VWFpp:Ag were measured and interpreted in conjunction with anti-VWF titers to assess the balance between VWF secretion and clearance during the evolution of this AVWS case. VWFpp is cleaved in the trans-Golgi but remains stored together with mature VWF in platelet α-granules and endothelial cell Weibel-Palade bodies in equimolar amounts.9 After release, VWFpp dissociates from the mature VWF subunit, circulates with a steady-state half-life, and serves as a marker of VWF secretion.10,11 In normal individuals and congenital VWD caused by decreased VWF biosynthesis, VWFpp:Ag is ∼1 (range, 0.9 - 1.45).12,13 VWFpp:Ag is increased when there is increased VWF clearance relative to secretion.12,13 For example, elevated VWFpp:Ag is seen in Vicenza-type VWD, where the decreased VWF level is a result of increased clearance, rather than defective VWF biosynthesis.12,13 VWFpp itself has also been proposed as a marker of endothelial cell perturbations, where VWFpp level reflects increased VWF secretion14
At diagnosis, the increased VWFpp with infinitely high VWFpp:Ag suggests VWF secretion was upregulated but was unable to compensate for the rapid clearance by anti-VWF antibody (Table 1). The raised C-reactive protein suggests autoimmune-mediated acute endothelial damage may have contributed to the increased VWF release (VWFpp). In RM1, VWFpp:Ag decreased with the falling anti-VWF titer, but remained ∼2 times above normal, consistent with continuing VWF clearance by the remaining anti-VWF (Figure 1B). The normal VWF/FVIII level and VWF-multimer pattern was therefore secondary to a compensatory increase in VWF secretion, as evidenced by a VWFpp level that remained as high as that at diagnosis.
Subsequently, RL1 occurred with absent VWF/FVIII level, despite anti-VWF antibody titer being similar to that at RM1 (Figure 1B). The VWFpp level had decreased to half that seen in RM1 (Table 1), suggesting that a fall in VWF secretion during RL1 allowed for phenotypic relapse, despite unchanged anti-VWF-mediated clearance. The idea of impaired VWF secretion is corroborated by the 1-deamino-8-D-arginine vasopressin (DDAVP) stimulation results at RL1 (Table 1). In certain anti-VWF-mediated AVWS cases, DDAVP was able to increase VWFpp levels by 5- to sevenfold,12,15 so that DDAVP may be used therapeutically.16,17 In our patient, there was no VWF:Ag increment and only a slight increase in VWFpp (1.33-fold) after DDAVP administration. The lower VWFpp at RL1 and less-than-expected increase in VWFpp in response to DDAVP may potentially be a result of an exhausted VWF/VWFpp pool resulting from chronic low-grade endothelial activation secondary to the partially treated underlying autoimmune disorder.13,14 Endothelial cell perturbations have been described in systemic lupus erythematous and during immunosuppressive therapies.18,19 Ultimately, the lower VWF/VWFpp secretion allowed the extremely rapid clearance by anti-VWF antibodies to dominate in RL1.
In our patient, CR was reached only when anti-VWF became undetectable and the VWFpp:Ag, in addition to VWF/FVIII, levels completely normalized.
By following-up our patient through several relapses and remissions, we were able to demonstrate how the interaction between VWF secretion and anti-VWF antibody titer plays a role in phenotypic status. VWF/FVIII levels by themselves were insufficient to provide true remission status, and absent anti-VWF antibody titer was needed to confirm CR. However, the anti-VWF antibody assay is challenging to standardize and not always available. Here we show the advantage of VWFpp:Ag in providing information on the balance between VWF secretion and clearance. This commercially available assay proved to be useful in predicting remission state at both RM1 and CR. The elevated VWFpp:Ag was consistent with an incomplete remission state at RM1, and the normal VWFpp:Ag confirmed CR status at a time when VWF/FVIII was normal and anti-VWF was absent. VWFpp:Ag has been suggested in other studies15,20,21 to be helpful in the diagnosis of AVWS, but to the best of our knowledge, none have followed VWFpp:Ag throughout the course of AVWS treatment. This case study suggests VWFpp:Ag may be an easy and reliable tool for monitoring and determining remission status in anti-VWF-mediated AVWS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Dr Adrienne Lee is a recipient of the Bayer International Hemophilia Scholarship Award. We thank Baxter Healthcare Corporation, Mississauga, ON, Canada, for generously providing recombinant VWF for the ELISA study. We also thank Angie Tuttle from the Clinical and Molecular Hemostasis Research Laboratory at Queen’s University, Kingston ON, Canada, for performing VWFpp:Ag assays and Deanna Kanderka from the Molecular Hematology Laboratory at Calgary Laboratory Services for performing anti-rVWF ELISA and multimer assays.
Authorship
Contribution: A.L., G.S., and M.-C.P. designed the study. G.S. supervised the anti-rVWF ELISA and multimer assays. P.J. oversaw the VWFpp assays. A.L. and M.-C.P. wrote the manuscript. All authors participated in its revision.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Man-Chiu Poon, Foothills Medical Centre, 1403 29th St NW, Calgary, AB, Canada T2N 2T9; e-mail: mcpoon@ucalgary.ca.