Key Points
Among patients with >10% BCR-ABL1, at 3 months, the poorest-risk group can be distinguished by the rate of BCR-ABL1 decline from baseline.
Patients with BCR-ABL1 values on a constant downward trajectory may rapidly reach the level considered optimal with additional follow-up.
Abstract
In chronic myeloid leukemia (CML) patients, a breakpoint cluster region–Abelson (BCR-ABL1) value >10% at 3 months of therapy is statistically associated with poorer outcome, yet many of these patients still achieve satisfactory outcomes. We investigated 528 first-line imatinib-treated patients to determine whether patients with the poorest outcome can be better discriminated at 3 months. All outcomes were significantly superior for the 410 patients with BCR-ABL1 ≤10% at 3 months (P < .001). However, the poorest outcomes among the 95 evaluable patients with BCR-ABL1 >10% at 3 months were identified by the rate of BCR-ABL1 decline from baseline, assessed by estimating the number of days over which BCR-ABL1 halved. Patients with BCR-ABL1 halving time <76 days (n = 74) had significantly superior outcomes compared with patients whose BCR-ABL1 values did not halve by 76 days (n = 21; 4-year overall survival, 95% vs 58%, P = .0002; progression-free survival, 92% vs 63%, P = .008; failure-free survival, 59% vs 6%, P < .0001; and major molecular response, 54% vs 5%, P = .008). By multivariate analysis, the halving time was an independent predictor of outcome in this poor risk group. Our study highlighted that the rate of BCR-ABL1 decline may be a critical prognostic discriminator of the patients with very poor outcome among those >10% at 3 months. The International Randomized IFN vs STI571 (IRIS) trial was registered at http://www.clinicaltrials.gov as #NCT00006343. The Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) trial was registered at http://www.clinicaltrials.gov as #NCT00124748. The Therapeutic Intensification in DE-novo Leukaemia (TIDEL) I trial was registered at http://www.ANZCTR.org.au as #ACTRN12607000614493. The TIDEL II trial was registered at http://www.ANZCTR.org.au as #ACTRN12607000325404.
Introduction
The molecular response at 3 months of tyrosine kinase inhibitor (TKI) therapy for patients with chronic myeloid leukemia (CML) has prognostic significance and has been confirmed by many groups.1-7 A breakpoint cluster region–Abelson (BCR-ABL1) transcript level >10% on the international reporting scale (IS) at 3 months is consistently associated with significantly inferior overall survival (OS), progression-free survival (PFS), failure-free survival (FFS), and cytogenetic and molecular responses. Marin et al2 and Neelakantan et al8 stated that measuring BCR-ABL1 at 3 months is the only requirement to predict outcome.
The compelling evidence supporting the importance of the initial molecular response for predicting outcome has led to the incorporation of milestone molecular responses into treatment decision algorithms published by the European LeukemiaNet (ELN)9 and the National Comprehensive Cancer Network (NCCN).10 To limit the risk of progression and death, treatment intervention is mandated when certain response criteria are not met. A point of divergence between the ELN and the NCCN is the timing of treatment intervention based on milestone BCR-ABL1 values. The NCCN guidelines include a change of therapy if BCR-ABL1 is >10% at 3 months, whereas the ELN suggests that a single BCR-ABL1 measurement at 3 months is insufficient to define treatment failure requiring a change of therapy. For patients with >10% BCR-ABL1 at 3 months, the ELN recommends additional testing and a therapy change for patients who are still >10% after 6 months of treatment.
In our cohort of 528 first-line imatinib-treated patients described here, we found that some patients with a BCR-ABL1 value >10% at 3 months achieved satisfactory outcomes. Our study aimed to find a better early discriminator of the poorest-risk patients at 3 months, which would refine treatment decisions based on the early molecular response. We found that the rate of decline of BCR-ABL1 from the individual patient baseline (pre-imatinib) value when measured at 3 months was strongly associated with significant differences in outcome. Patients with minimal or no decline from the baseline value had inferior outcomes. Among patients with >10% BCR-ABL1 at 3 months, assessing the rate of BCR-ABL1 decline compared with the pre-imatinib value could aid decisions regarding the timing of therapeutic intervention.
Patients and methods
Patient population
Between July 2000 and March 2011, 528 patients with newly diagnosed chronic phase CML were enrolled in consecutive clinical trials of 400, 600, or 800 mg of imatinib daily and were monitored by peripheral blood molecular analysis at our institution. These trials included a subset of patients from the Novartis-sponsored International Randomized IFN vs STI571 (IRIS) trial (n = 29, all patients enrolled in Australia and New Zealand);11 a subset of patients from the Novartis-sponsored Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) trial (n = 186, all patients enrolled in Australia, New Zealand, Singapore, South Africa, and South America);12 and patients enrolled in the Australasian Leukaemia and Lymphoma Group Therapeutic Intensification in DE-novo Leukaemia (TIDEL) I (n = 103)13 and TIDEL II studies (n = 210).14 Results were included until the time of last molecular follow-up of each patient (cutoff May 20, 2013). The minimum elapsed time since commencing imatinib for all patients was 27 months, and the median time on therapy was 39 months (range, 1-149 months).
Imatinib was the only therapy in all patients during the first 3 months. In 2 studies, failure to achieve time-dependent milestone molecular responses led to an imatinib dose increase (TIDEL I and TIDEL II) or a switch to nilotinib (TIDEL II). The current analysis does not include an assessment of the impact of early therapeutic switch on response, which will be addressed elsewhere for TIDEL II (D.T.Y., T.P.H., and A.P.G., unpublished data, 2014). During the time of molecular follow-up of the total cohort, a switch to nilotinib occurred in 79 patients (median month of switch was 7, range 3-84 months), dasatinib in 9 patients, and ponatinib in 1. All trials were conducted according to the Declaration of Helsinki, with written informed consent, and approved by national/international ethics committees.
Molecular analysis
The method for measuring BCR-ABL1 transcripts was described previously.15 BCR as the control gene has been studied extensively for suitability for BCR-ABL1 measurement15-20 and was used in this study. We previously demonstrated the consistency and measurement reliability of our method for the quantification of BCR-ABL1.15-17,19 The results were reported as BCR-ABL1/BCR% IS.19 BCR-ABL1 values in this study were converted to the IS using our laboratory specific conversion factor: 1.25.19 Molecular monitoring was performed prior to commencing imatinib (baseline), at 1, 2, and 3 months, and every 3 to 6 months thereafter. The exception was patients enrolled in the IRIS trial where 1- and 2-month sample collections were omitted; however, the baseline, 3-month, and every 3- to 6-month sample collections were included as part of an IRIS trial preplanned substudy.21 The achievement of a major molecular response (MMR; ≤0.10% IS) and molecular response 4.5 (MR4.5; ≤0.0032% IS) required confirmation at 2 consecutive measurements.
Halving time calculation
The rate of BCR-ABL1 change from each patient’s baseline value was assessed at 1, 2, and 3 months of imatinib by estimating the number of days required for BCR-ABL1 to achieve one-half of the baseline value, termed the halving time. Calculated as c = −ln(2)/k, where c is the halving time and k is the fold BCR-ABL1 change from the baseline value divided by the number of days after the imatinib starting day (day zero) of the 1-, 2-, or 3-month BCR-ABL1 measurement. k = [ln(b) − ln(a)]/d, where a is the baseline BCR-ABL1 value, b is the BCR-ABL1 value at the relevant time point, and d is the number of days between measurements.
To determine the validity of assessing response kinetics with an exponential decline model for halving time, log10BCR-ABL1 values were plotted against the number of days between measurements for 485 patients with ≥3 measurements in the first 3 months. An exponential relationship in such a semi-log format will show a linear relationship between the variables (reflecting the constant rate of change), and therefore, the linear correlation was assessed using the Pearson correlation coefficient. Additionally, method-dependent nonlinearity may occur at BCR-ABL1 values >10% IS.22-24 Therefore, we assessed the linear relationship separately in patients with multiple BCR-ABL1 measurements and values >10% or ≤10% (supplemental Results available on the Blood Web site).
For patients with a constant BCR-ABL1 decline, the value on any given day can be estimated using a formula derived from the halving time formula, b = a × 2(−d/c), where b is the estimated BCR-ABL1 value based on the 3-month halving time, a is the baseline BCR-ABL1 value, d is the number of days between imatinib start and sample collection, and c is the halving time calculated at 3 months.
Statistical analysis
Cumulative incidence curves for the achievement of molecular responses were calculated according to recommendations.25,26 An event was the achievement of the molecular response of interest and competing risks included all permanent discontinuations of TKI for any reason, other than completion of study protocol. Fine and Gray models implemented in R27,28 were used to examine the association between each of the baseline risk factors and the molecular responses.29,30 Relative risks and their 95% confidence intervals (CIs) were calculated from these regression models, and significance was determined with the Wald test. The Akaike information criterion was used for model selection in the multivariate Fine and Gray regression.
Survival analyses were performed using the Kaplan-Meier method.31 Events were defined for OS, PFS (accelerated phase or blast crisis [BC]), and FFS according to ELN recommendations.9,26 Failure events included lack of milestone responses at 3, 6, and 12 months, loss of hematologic, cytogenetic, or molecular response, acquisition of BCR-ABL1 mutations, clonal chromosomal abnormalities in Ph+ cells, progression to accelerated phase or BC, and death. Survival probabilities were compared using the log-rank test. Hazard ratios were derived using the Cox proportional hazard model.32
Receiver operating characteristic (ROC) curves were generated using the pROC statistical package for R.33 Optimal thresholds along the ROC curves were calculated using the Youden index. Correlation coefficients were compared between BCR-ABL1 values ≤10% and >10% using the Mann-Whitney test and between the baseline BCR-ABL1 quartile groups using the Kruskal-Wallis analysis of variance. The effect of hydroxyurea treatment and the timing of the baseline measurement on the baseline BCR-ABL1 values were assessed using Kruskal-Wallis. The paired t test was used to assess baseline BCR-ABL1 values before and after hydroxyurea therapy.
Results
BCR-ABL1 value at 3 months predicted outcome
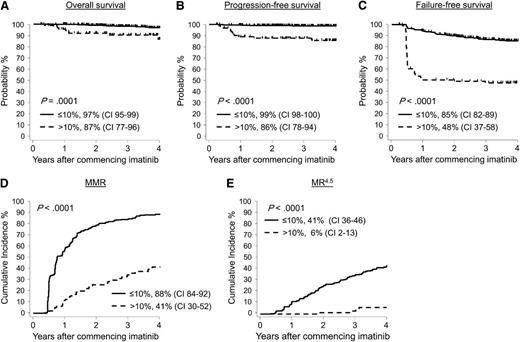
Of the total 528 patients, 507 had a BCR-ABL1 assessment at 3 months and were included in the analysis; 18 had missing data, 2 died, and 1 progressed to BC at 2 months. Consistent with other studies, responses were significantly superior for patients with BCR-ABL1 values ≤10% (n = 410, 78% of all patients) compared with those with >10% (n = 97, 18% of all patients). After 4 years from commencement of imatinib, the outcomes comparing ≤10% vs >10% were as follows: OS, 97% vs 87%, P = .0001; PFS, 99% vs 86%, P < .0001; FFS, 85% vs 48%, P < .0001; MMR, 88% vs 41%, P < .0001; and MR4.5, 41% vs 5.7%, P < .0001 (Figure 1). Of the 97 patients with BCR-ABL1 >10% at 3 months, 1 was already in BC and 13 subsequently progressed, 11 of which occurred before 12 months. Thus, transformation for patients >10% at 3 months was high (14/97 patients, 14%), and the events usually occurred within the first year of therapy (11/14, 79%).
Patients with BCR-ABL1 values ≤10% at 3 months had significantly better outcome than patients with BCR-ABL1 values >10%. A BCR-ABL1 value >10% at 3 months is categorized as a warning or treatment failure and occurred in 97 of 528 patients. These patients had significantly inferior (A) OS, (B) PFS, (C) FFS, (D) MMR, and (E) MR4.5 compared with patients where BCR-ABL1 was ≤10% at 3 months (n = 410).
Patients with BCR-ABL1 values ≤10% at 3 months had significantly better outcome than patients with BCR-ABL1 values >10%. A BCR-ABL1 value >10% at 3 months is categorized as a warning or treatment failure and occurred in 97 of 528 patients. These patients had significantly inferior (A) OS, (B) PFS, (C) FFS, (D) MMR, and (E) MR4.5 compared with patients where BCR-ABL1 was ≤10% at 3 months (n = 410).
The initial starting dose of imatinib led to differences in the percentage of patients with >10% BCR-ABL1 at 3 months. Of the 83 evaluable patients with an initial starting dose of 400 mg, 25 (30%) had BCR-ABL1 values >10% at 3 months. In contrast, 47 of 303 (16%) and 25 of 121 (21%) of patients treated with 600 or 800 mg, respectively, had BCR-ABL1 values >10% at 3 months. However, there was no statistical difference in outcome between the dose groups (Table 1).
The significantly inferior outcome for patients with BCR-ABL1 >10% at 3 months is consistent with the report by Marin et al.2 That study performed ROC analysis to determine optimal threshold values to predict response. We performed a similar analysis and the optimal threshold values were comparable for the same outcomes: OS, BCR-ABL1 16.1% (area under the curve [AUC], 0.66; 95% CI, 0.5-0.81; Marin et al2 threshold BCR-ABL1 9.84%); PFS, 9.56% (AUC, 0.72; 95% CI, 0.61-.83; Marin et al2 threshold 9.54%); and FFS, 8.44% (AUC, 0.76; 95% CI, 0.71-0.81). Marin et al2 assessed event-free survival, which is not comparable with the definition of FFS used in our analysis. The optimal threshold in our analysis to achieve MMR by 12 months was a BCR-ABL1 value of 1.45% at 3 months (AUC, 0.84; 95% CI, 0.81-0.88) and MMR by 4 years was 6.58% (AUC, 0.83; 95% CI, 0.79-0.87).
Subgroup of patients with the poorest outcome was identified at 3 months by the rate of BCR-ABL1 decline from imatinib start
Notwithstanding the usefulness of the 3-month value of >10% for outcome prediction, there was a sizeable number of patients within this subgroup who did not fail therapy and some subsequently reached an optimal response despite being initially classified as a poor responder at 3 months. Thirty-four of 97 patients with >10% at 3 months achieved an MMR, which was maintained in 28 patients (82%) at last follow-up (median, 26 months after MMR achieved; range, 3-103 months). We investigated whether the patients with the poorest outcomes among those with >10% at 3 months could be identified.
The kinetics of the BCR-ABL1 decline from the individual patient baseline value to the 3-month time point was examined. We based this examination on the observation that some patients with >10% at 3 months had very little or no decline, whereas others had more than a 30-fold reduction. Furthermore, we found that patients with the same BCR-ABL1 value at 3 months had better outcomes, on average, if their baseline value was higher. This suggested that the rate of BCR-ABL1 decline after commencing imatinib may be important for outcome. The rate of BCR-ABL1 change was examined by estimating the BCR-ABL1 halving time from the individual patient baseline value. Longer halving times indicate a slow, or no, reduction of BCR-ABL1. Halving times were also calculated for the patients with <10% BCR-ABL1 at 3 months. There was only 1 of 410 patients where we observed no reduction of BCR-ABL1 value at 3 months from baseline; hence, this patient had a long halving time.
The halving time calculation incorporates the relative BCR-ABL1 change between measurements and the number of days between measurements, which are both important considerations for reliable assessment of the kinetics of response.16,34,35 The halving time calculation assumes a constant rate of change, which was found to be a valid assumption using our method (supplemental Results; supplemental Table 1). Furthermore, this relationship was not affected when BCR-ABL1 values were outside of the IS effective measurement range (supplemental Results; supplemental Table 2). Other factors that we investigated that could potentially influence the BCR-ABL1 value at baseline, and hence the halving time, were the number of days between the baseline measurement and the imatinib starting date and prior hydroxyurea therapy. The duration between the baseline BCR-ABL1 measurement and the day of starting imatinib did not affect BCR-ABL1 values (range, 1-50 days; supplemental Results; supplemental Table 3). Similarly, the number of days of prior hydroxyurea therapy had no effect on the baseline values (range, 1-325 days) (supplemental Results; supplemental Table 4).
We evaluated the discriminatory power for outcome prediction of the BCR-ABL1 halving time at 3 months among the patients with >10% at 3 months. Where there was no BCR-ABL1 reduction from baseline at 3 months, the halving times were negative. This occurred in 9 patients. To enable assessment of the discriminatory power of the BCR-ABL1 halving time, the halving times of these 9 patients were imputed to the longest positive halving time of 2000 days, which was calculated for the patient with the smallest decline. Using ROC analysis, the optimal halving time thresholds for discriminating between outcomes were as follows: OS, 63 days (AUC, 0.77; 95% CI, 0.51-0.85); PFS, 76 days (AUC, 0.68; 95% CI, 0.51-0.85); FFS, 50 days (AUC, 0.79; 95% CI, 0.70-0.88); and MMR, 43 days (AUC, 0.74; 95% CI, 0.65-0.84). Of the highly relevant outcomes OS and PFS, we selected the optimal PFS halving time of 76 days as a classifier for further outcome prediction because this measure was independent of death due to non–CML-related causes in our cohort.
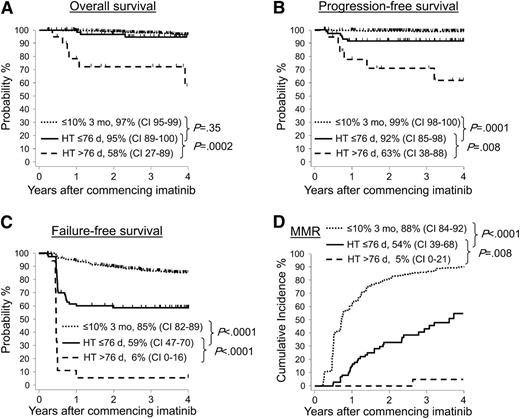
Of the patients with BCR-ABL1 >10% at 3 months, those where BCR-ABL1 had declined from their baseline value by at least one-half by 76 days (74/95 evaluable patients, 78%) had significantly superior outcomes compared with the 21 of 95 patients (22%) where the halving time was >76 days (OS, 95% vs 58%, P = .0002; PFS, 92% vs 63%, P = .008; FFS, 59% vs 6%, P < .0001; and MMR, 54% vs 5%, P = .008; Figure 2). Only 1 patient with a halving time >76 days did not subsequently meet an ELN criterion for failure, and only 1 achieved MMR. Figure 3 shows the change in BCR-ABL1 values between baseline and the day of collection of the 3-month sample for each patient with >10% BCR-ABL1 at 3 months, according to the halving time calculated at 3 months.
Patients with BCR-ABL1 values >10% at 3 months had better outcomes if the BCR-ABL1 halving time was ≤76 days. Among the 95 patients with BCR-ABL1 values >10%, the 74 patients with a halving time of ≤76 days had significantly superior (A) OS, (B) PFS, (C) FFS, and (D) MMR compared with the 21 patients with a halving time of >76 days. For some of the patients with an assigned halving time of >76 days, their BCR-ABL1 value did not halve at any time or increased. The outcome for patients with BCR-ABL1 values ≤10% at 3 months are also plotted.
Patients with BCR-ABL1 values >10% at 3 months had better outcomes if the BCR-ABL1 halving time was ≤76 days. Among the 95 patients with BCR-ABL1 values >10%, the 74 patients with a halving time of ≤76 days had significantly superior (A) OS, (B) PFS, (C) FFS, and (D) MMR compared with the 21 patients with a halving time of >76 days. For some of the patients with an assigned halving time of >76 days, their BCR-ABL1 value did not halve at any time or increased. The outcome for patients with BCR-ABL1 values ≤10% at 3 months are also plotted.
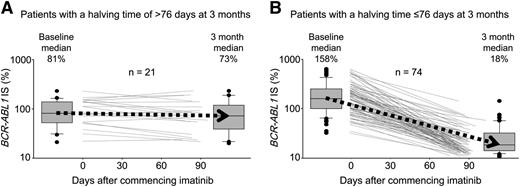
Change in BCR-ABL1 value from the individual patient baseline value according to the halving time at 3 months for patients with >10% BCR-ABL1. (A) Twenty-one patients had a halving time >76 days and (B) 74 patients had a halving time ≤76 days. The lines represent the change in BCR-ABL1 value from baseline to the day of collection of the 3-month sample. The box plots represent the median and interquartile range of the baseline and 3-month measurements. The response outcome probabilities were inferior for patients with little or no change.
Change in BCR-ABL1 value from the individual patient baseline value according to the halving time at 3 months for patients with >10% BCR-ABL1. (A) Twenty-one patients had a halving time >76 days and (B) 74 patients had a halving time ≤76 days. The lines represent the change in BCR-ABL1 value from baseline to the day of collection of the 3-month sample. The box plots represent the median and interquartile range of the baseline and 3-month measurements. The response outcome probabilities were inferior for patients with little or no change.
Twenty-one of 22 patients with a halving time at 3 months of >76 days in our total cohort also had >10% BCR-ABL1 at 3 months. The exception was a patient with a low BCR-ABL1 value of 3.7% at baseline, followed by a slow increase after starting imatinib and a protocol-mandated switch to nilotinib at 6 months due to a BCR-ABL1 value >10% (ELN failure criterion).
BCR-ABL1 halving time calculated at 3 months was an independent predictor of outcome among patients with >10% BCR-ABL1 at 3 months
For the patients with >10% BCR-ABL1 at 3 months, the prognostic value of the BCR-ABL1 halving time at 3 months for OS, PFS, FFS, and MMR was compared with the baseline variables listed in Table 1. A halving time calculated at 3 months of >76 days was the only variable that significantly predicted for each of the outcome measures by univariate analysis and in the multivariate regression model (Table 2).
Timing of the 3-month assessment potentially changes the interpretation of response
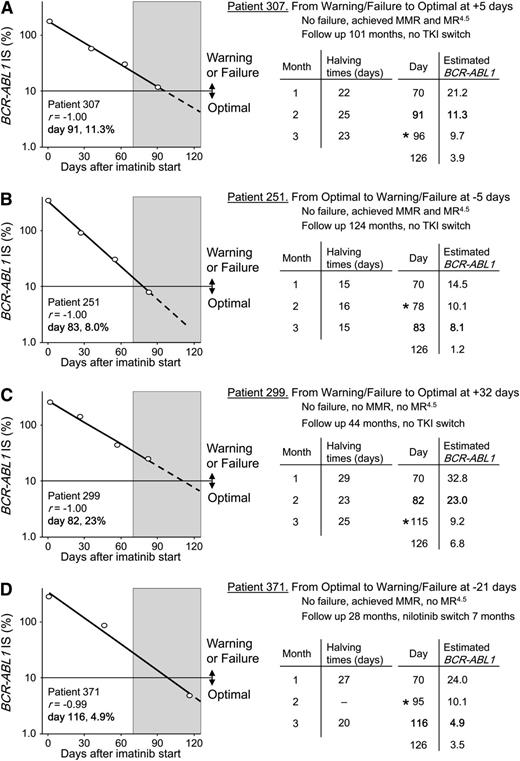
For some patients with >10% BCR-ABL1 at 3 months and a constant rate of decline from baseline, a shift in the day of the 3-month sample collection could theoretically change the response category from warning or failure to optimal and vice versa. This is important because measurements classified as 3 months could span a wide collection window from the TKI starting day.25 The 3-month samples in our cohort were collected from days 70 to 126 (median, 86 days), spanning a window of 56 days. To demonstrate how response classifications at 3 months could alter by shifting the day of collection, the BCR-ABL1 value on any given day was estimated from the rate of the BCR-ABL1 decline. Figure 4 shows 4 patient examples where the 3-month milestone response category would change if the day of collection of the 3-month sample was shifted by as little as 5 days. This analysis demonstrates that patients with BCR-ABL1 values on a constant downward trajectory may reach the level considered optimal with additional follow-up, particularly if the BCR-ABL1 value is close to the 10% threshold value for determining the response category.
Predicted change in 3-month response category according to the day of sample collection. A BCR-ABL1 value of 10% at 3 months discriminates between treatment failure or a warning and an optimal response according to current recommendations and guidelines. (A-D) Plots of the actual BCR-ABL1 decline for 4 patients with multiple BCR-ABL1 measurements within the first 3 months of imatinib treatment. The circles are the actual BCR-ABL1 values of the sample collections at baseline and the 1-, 2-, and 3-month time points. The decline was exponential in each patient as indicated by the correlation coefficient of the regression line r. The actual day of the 3-month collection from the imatinib start date and the actual BCR-ABL1 values are indicated within each graph in bold. The shaded region represents the 56-day measurement window over which the 3-month samples in our cohort were collected (days 70-126). The dashed lines represent the continuation of the regression line within the measurement window. The halving times at 1, 2, and 3 months were each calculated using the individual patient’s baseline value. We estimated the BCR-ABL1 values for the extremes of the measurement window (day 70 and day 126) for each patient, using the known 3-month halving time, the patient’s baseline BCR-ABL1 value, and by varying the day of sample collection in the formula. This assumes the decline remains constant within the measurement window. The actual and estimated BCR-ABL1 values for each patient on the actual day of the 3-month sample collection were almost identical in each case, which are indicated in bold text. The asterisk indicates the collection day where the response classification would change.
Predicted change in 3-month response category according to the day of sample collection. A BCR-ABL1 value of 10% at 3 months discriminates between treatment failure or a warning and an optimal response according to current recommendations and guidelines. (A-D) Plots of the actual BCR-ABL1 decline for 4 patients with multiple BCR-ABL1 measurements within the first 3 months of imatinib treatment. The circles are the actual BCR-ABL1 values of the sample collections at baseline and the 1-, 2-, and 3-month time points. The decline was exponential in each patient as indicated by the correlation coefficient of the regression line r. The actual day of the 3-month collection from the imatinib start date and the actual BCR-ABL1 values are indicated within each graph in bold. The shaded region represents the 56-day measurement window over which the 3-month samples in our cohort were collected (days 70-126). The dashed lines represent the continuation of the regression line within the measurement window. The halving times at 1, 2, and 3 months were each calculated using the individual patient’s baseline value. We estimated the BCR-ABL1 values for the extremes of the measurement window (day 70 and day 126) for each patient, using the known 3-month halving time, the patient’s baseline BCR-ABL1 value, and by varying the day of sample collection in the formula. This assumes the decline remains constant within the measurement window. The actual and estimated BCR-ABL1 values for each patient on the actual day of the 3-month sample collection were almost identical in each case, which are indicated in bold text. The asterisk indicates the collection day where the response classification would change.
Discussion
This study confirmed the significant difference in outcome for patients with >10% BCR-ABL1 at 3 months of therapy compared with those with ≤10%,2-7 which further supports the incorporation of the 10% BCR-ABL1 level in treatment recommendations and guidelines.9,10 However, our study also demonstrated that not all imatinib-treated patients with >10% at 3 months have unsatisfactory outcomes and that the degree of BCR-ABL1 change from the individual patient baseline value at 3 months is highly informative for the prediction of outcome. Among the 18% of all patients in our cohort with >10% at 3 months, lack of a BCR-ABL1 decline or a slow decline from baseline conveyed the highest risk of treatment failure, progression, and death. This subgroup consisted of 21 patients (4% of those commencing imatinib). In contrast, those patients with a more rapid decline had a high chance of ultimately achieving MMR and hence an optimal response.
To capture differences in the rate of decline between patients, we calculated the BCR-ABL1 halving time at 3 months. The halving time demonstrated independent prognostic value in the multivariate analysis. Halving times of tumor markers in response to chemotherapy have been used to distinguish prognostic subgroups for a variety of tumor types.36-42 Slower rates of marker decline were consistently associated with the poorest outcomes, which is consistent with our findings using BCR-ABL1 halving times. Two major factors affect the halving time: the decrease in BCR-ABL1 values expressed as a fold change from baseline and the number of days that elapsed between commencement of imatinib and the day of the 3-month measurement. Using the BCR-ABL1 doubling time, which is an analogous method for assessing response kinetics,16,35 we have previously demonstrated that incorporating these 2 parameters can reveal major differences in kinetics, which may not be evident from the fold change alone.34
It is not our intention to provide definitive thresholds on which to base therapeutic decisions for patients with >10% BCR-ABL1 at 3 months, nor to recommend that formal calculations of halving times be included as part of routine molecular monitoring. Given that different assays are used to measure BCR-ABL1 transcripts and that there is method-dependent nonlinearity above 10% depending on the control gene,23,24 these parameters might not be generally applicable. Furthermore, we did not investigate whether there is an absolute value >10% BCR-ABL1 at 3 months that identified the poorest-risk patients using our method with BCR as the control gene, because there is limited opportunity for validation due to small number of study cohorts for which BCR-ABL1 values are calculated using BCR as the control. Our intention is to emphasize that outcomes are heterogeneous among the patients with >10% at 3 months and that we can identify a subgroup at the highest risk of treatment failure. Failure of the BCR-ABL1 value to approximately halve at the 3-month measurement may help identify the poorest risk patients. This concept may equally apply for response to other TKIs, although the time at which BCR-ABL1 must halve may be earlier. Additional molecular tests in the first 3 months of therapy could be helpful for response prediction.
Consistent with our findings, Hanfstein et al recently measured the decline of BCR-ABL1 over the first 3 months of imatinib using beta-glucuronidase as the control.43 Irrespective of the 3-month BCR-ABL1 value, a small cohort was identified with slower declines, as indicated by lesser fold reductions of BCR-ABL1 from the baseline value. These patients had the poorest outcomes. The authors suggested that this assessment may identify poor risk patients more precisely than the actual 3-month value.43 For patients with little or no change of BCR-ABL1 from baseline, methods using ABL1 as the control might also provide similar prognostic information for risk stratification.
In our cohort of patients enrolled in clinical trials, the 3-month samples were collected over a 56-day window. Allocation to the 3-month time point is suggested for samples collected within the range of 1.5 to 4.5 months25 : a >90-day window for this critical milestone time point. By modeling BCR-ABL1 values for individual patients over different 3-month sample collection days, we found that the response classification for patients with BCR-ABL1 values that are on a constant downward trajectory over the first 3 months could change from warning9 or failure10 to optimal, or vice versa, depending on the timing of sample collection within the 3-month window (Figure 4). This initial phase of therapy is when the most rapid decline of BCR-ABL1 occurs44-48 and is before a second, slower decline occurs in many patients.44,45 Our theoretical demonstration may serve as a caution for clinicians when assessing the 3-month response, particularly if the BCR-ABL1 value is >10%, and the sample is collected at the earliest extreme of the 3-month assessment window.
We confirmed the robustness of our observations using BCR-ABL1 halving times to assess response by carefully ensuring that the BCR-ABL1 decline was linear both above and below the threshold of the IS using our method. We also demonstrated that the timing of the baseline measurement with respect to when imatinib treatment actually started did not alter the pre-imatinib BCR-ABL1 values and found no effect on pre-imatinib BCR-ABL1 values if hydroxyurea had been used prior to imatinib treatment.
The NCCN guidelines include a change of therapy at 3 months based on a single BCR-ABL1 assessment, in contrast to the more cautious approach of the ELN, which does not recommend a change of therapy based on BCR-ABL1 values until 6 months. This lack of consensus may create a dilemma for clinicians when considering the timing and necessity of therapeutic intervention. A change of therapy may have implications in terms of additional cost and potential long-term toxicity. Data suggest that nilotinib is associated with a higher rate of vascular events,49,50 dasatinib therapy with pleural effusions and pulmonary arterial hypertension,51 and ponatinib with pancreatitis and a high rate of vascular events.52 Our study highlights the importance of performing molecular analysis prior to commencing TKI therapy to assess the BCR-ABL1 decline over the critical first 3 months. An examination of the initial rate of decline by a sequence of molecular tests over the first 3 months may provide a cost-effective process for the better identification of patients for whom the risks and potential additional drug costs of therapy change are justified.
In conclusion, our study demonstrated that the rate of BCR-ABL1 decline from baseline may be a critical prognostic discriminator of the very poor prognosis patients among those who are >10% at 3 months. This could help to refine recommendations for treatment decisions at early time points.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the many patients, clinicians, and study coordinators who contributed samples and follow-up data to this study and the Centre for Cancer Biology–An Alliance between SA Pathology and the University of South Australia.
This work was supported by the Australasian Leukaemia and Lymphoma Group and Novartis, including research support to S.B., T.P.H., D.T.Y., and D.M.R. D.T.Y. is supported by a scholarship from the Leukaemia Foundation of Australia and the AR Clarkson Foundation. W.T.P. is funded by a postdoctoral fellowship from the Leukaemia Foundation of Australia/Cure Cancer Australia.
Authorship
Contribution: S.B. designed and performed the research, analyzed data, and wrote the manuscript; D.T.Y. analyzed data, contributed to the experimental design, and contributed to manuscript preparation; T.P.H., W.T.P., and L.P. contributed to the experimental design and contributed to manuscript preparation; N.D.R. analyzed data and contributed to the manuscript preparation; J.F.S., A.P.G., and D.M.R. contributed to the manuscript preparation; and J.A.B., H.K.A., A.L.Y., J.G., B.A.J., S.P., Z.D., M.L., and L.F. performed research and contributed to manuscript preparation.
Conflict-of-interest disclosure: S.B. and T.P.H. are advisory board members and have received research funding and honoraria from Novartis, Bristol-Myers Squibb, and Ariad. D.T.Y. has received research funding from Novartis and Bristol-Myers Squibb. D.M.R. has been an advisory board member and received research funding and honoraria from Novartis and honoraria from Bristol-Myers Squibb. J.F.S. is an advisory board member and has received honoraria from Novartis and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Susan Branford, Department of Genetics and Molecular Pathology, SA Pathology, PO Box 14 Rundle Mall, Adelaide, SA 5000, Australia; e-mail: susan.branford@health.sa.gov.au.