Key Points
Carfilzomib, rituximab, and dexamethasone (CaRD) produce overall and CR/VGPR responses in 87% and 36% of frontline WM patients, respectively.
CaRD activity was not impacted by MYD88 and CXCR4 mutations and represents a neuropathy-sparing option for treating WM patients.
Abstract
Bortezomib frequently produces severe treatment-related peripheral neuropathy (PN) in Waldenström’s macroglobulinemia (WM). Carfilzomib is a neuropathy-sparing proteasome inhibitor. We examined carfilzomib, rituximab, and dexamethasone (CaRD) in symptomatic WM patients naïve to bortezomib and rituximab. Protocol therapy consisted of intravenous carfilzomib, 20 mg/m2 (cycle 1) and 36 mg/m2 (cycles 2-6), with intravenous dexamethasone, 20 mg, on days 1, 2, 8, and 9, and rituximab, 375 mg/m2, on days 2 and 9 every 21 days. Maintenance therapy followed 8 weeks later with intravenous carfilzomib, 36 mg/m2, and intravenous dexamethasone, 20 mg, on days 1 and 2, and rituximab, 375 mg/m2, on day 2 every 8 weeks for 8 cycles. Overall response rate was 87.1% (1 complete response, 10 very good partial responses, 10 partial responses, and 6 minimal responses) and was not impacted by MYD88L265P or CXCR4WHIM mutation status. With a median follow-up of 15.4 months, 20 patients remain progression free. Grade ≥2 toxicities included asymptomatic hyperlipasemia (41.9%), reversible neutropenia (12.9%), and cardiomyopathy in 1 patient (3.2%) with multiple risk factors, and PN in 1 patient (3.2%) which was grade 2. Declines in serum IgA and IgG were common. CaRD offers a neuropathy-sparing approach for proteasome inhibitor-based therapy in WM. This trial is registered at www.clinicaltrials.gov as #NCT01470196.
Introduction
The proteasome inhibitor bortezomib is active in Waldenström’s macroglobulinemia (WM),1 with single agent responses in 60% to 70% of untreated or relapsed/refractory WM patients and response durations of 8 to 16 months.2-4 In combination with rituximab and dexamethasone, an overall response rate (ORR) of 95% was observed for untreated patients in a study by the WM Clinical Trials Group (WMCTG). Very good partial responses (VGPRs) or better occurred in 35% of patients, and the median progression-free survival (PFS) exceeded 4 years.5 Unfortunately, 70% of patients developed grade >2 peripheral neuropathy (PN) with the twice weekly dosing of bortezomib in this study, which resulted in premature bortezomib discontinuation in 60% of patients.5
The use of weekly bortezomib represented one of the first attempts to decrease PN risk in WM.6-8 Grade 3 PN occurred in 5% to 20% of patients and resulted in bortezomib discontinuation in 10% to 20% of subjects. Lower rates of VGPR or better accompanied weekly bortezomib use in some studies, a finding with possible implications for long-term disease control.9 In a hybrid study by the European Myeloma Network, patients received twice weekly bortezomib for the first cycle and then weekly bortezomib for cycles 2 to 5 with dexamethasone and rituximab. An ORR of 85%, with VGPR or better in 10% of patients, was observed.10 The median PFS was 43 months, and patients with VGPR/complete response (CR) had longer PFS. Grade ≥2 treatment-related PN occurred in 24% of patients and led to bortezomib discontinuation in 8% of patients.
Treatment-related PN with bortezomib can persist long after discontinuance of therapy and often requires the chronic use of gabapentin, pregabalin, and/or narcotics. The higher incidence of bortezomib-related PN in WM may reflect underlying nerve damage due to paraprotein-mediated demyelination and/or amyloid deposition that commonly occurs in WM.11,12 Carfilzomib is a second-generation proteasome inhibitor with a low neuropathic risk, including patients with baseline PN.13 Differences in off-target (nonproteasome) effects between bortezomib and carfilzomib have been proposed to account for the lower neuropathic potential of carfilzomib.14 In vitro, carfilzomib selectively inhibits the chymotrypsin-like activity of both the constitutive- and immuno-proteasome, the latter exhibiting higher expression in WM vs normal B cells and representing a selective target for WM-cell killing.15 As such, we evaluated the combination of carfilzomib, rituximab, and dexamethasone (CaRD) in patients with symptomatic WM. We also evaluated the influence of MYD88L265P and CXCR4WHIM somatic mutation status on response outcome given their high prevalence in WM patients, association with WM disease-related clinical features, and potential impact on treatment response.16-18
Patients and methods
This was a prospective, open-label, single stage, phase 2 study that enrolled patients at the Dana-Farber Cancer Institute (Boston, MA). All patients provided informed written consent, and the Institutional Review Board of the Harvard/Dana-Farber Cancer Center granted study approval. The study was conducted in accordance with the Declaration of Helsinki. Carfilzomib was supplied by Onyx Pharmaceuticals. Rituximab and dexamethasone were commercially obtained. The primary objective of the study was the determination of OR rate (ie, CR, VGPR, partial response [PR], and minimal response [MR] rates). Secondary objectives were determination of time to progression, as well as safety and tolerability including incidence of treatment peripheral neuropathy following CaRD therapy.
Symptomatic WM patients requiring therapy based on consensus recommendations,19 who were previously untreated with a proteasome inhibitor or rituximab, and had no more than 1 prior therapy were eligible. Intended therapy consisted of intravenous (IV) carfilzomib, 20 mg/m2, infused over 20 minutes (cycle 1 only) and then 36 mg/m2 infused over 30 minutes (for cycles 2 and beyond), with IV dexamethasone, 20 mg, given on days 1, 2, 8, and 9, and rituximab, 375 mg/m2, on days 2 and 9 every 21 days for 6 cycles of induction. Induction for patients with stable disease or better was followed by maintenance therapy 8 weeks later with IV carfilzomib, 36 mg/m2, and IV dexamethasone, 20 mg, given on days 1 and 2, along with rituximab, 375 mg/m2, on day 2 only every 8 weeks for 8 cycles. Rituximab was administered after carfilzomib and dexamethasone. For the first cycle only, treatment was administered after 1 L of saline to prevent azotemia. For grade >3 nonhematological toxicity, carfilzomib was held until toxicity was grade 1 or less, with dose reduction then permitted to 27 mg/m2 (level −1) for the first event and 20 mg/m2 (level −2) for the second event after cycle 1. For hematological toxicities, carfilzomib was held for an absolute neutrophil count <500/mm3 or platelet count <30 000/mm3. Filgrastim or transfusional support was permitted. Retreatment at the full dose of carfilzomib was permitted after the first occurrence of a drug hold for hematological toxicity, but thereafter, reduction to level −1 for the second event and level −2 for the third event was required. For grade ≥2 treatment-related neuropathy with pain, or grade 3 neuropathy without pain, treatment was held until neuropathy resolved to less than grade 2 without pain, followed by a decrease in carfilzomib to level −1 for the first event and level −2 for the second event. For grade 4 neuropathy, carfilzomib discontinuance was required.
For patients experiencing rituximab or dexamethasone intolerance, their discontinuation was permitted. Concomitant medications included acyclovir, 400 mg, twice a day for the duration of therapy plus 6 months for herpes zoster prophylaxis, and famotidine, 20 mg, twice a day during weeks of active therapy for prophylaxis against dexamethasone-related gastrointestinal toxicity. Dexamethasone (10 mg) was recommended the night before rituximab administration to attenuate risk of infusion reactions. The use of green tea or extracts was discouraged given potential adverse interactions with proteasome inhibitors.20 As per consensus and National Comprehensive Cancer Network guidelines, prophylactic use of plasmapheresis and hold on rituximab administration for patients demonstrating an immunoglobulin M (IgM) level of >4000 mg/dL prior to the administration of rituximab was recommended.21,22
Baseline studies consisted of complete blood counts and differential, quantitative serum IgM levels, serum protein electrophoresis, a bone marrow (BM) biopsy and aspiration, computed tomography (CT) scans of the chest, abdomen, and pelvis, serum electrolytes, liver function tests, amylase, lipase, blood urea nitrogen, creatinine, and serum β2-microglobulin levels. Patients were assessed for efficacy and toxicity on first day of induction cycles 2 to 6; at interphase, which occurred within 4 ± 2 weeks after the end of induction therapy; on first day of maintenance cycles 1 to 8; at conclusion of maintenance therapy, which occurred with 4 ± 2 weeks following last maintenance cycle; and thereafter off therapy every 12 ± 2 weeks. Required laboratories included complete blood counts and differential, quantitative serum IgM levels, serum protein electrophoresis, liver function tests, amylase, lipase, blood urea nitrogen, and creatinine on day 1 of each treatment cycle, interphase, end of maintenance, and during follow-up visits. At interphase and the end of maintenance visits, a BM biopsy and aspiration and CT scans of the chest, abdomen, and pelvis if extramedullary disease was present at baseline were required.
MYD88L265P and CXCRWHIM mutation genotyping
An allele-specific polymerase chain reaction (PCR) assay was used for determination of MYD88L265P using DNA isolated from CD19-selected BM or peripheral blood (PB) cells as previously described.23,24 CXCR4WHIM mutation status was determined by Sanger sequencing of CD19-selected BM cells as before.16,17
Statistical analysis
It was hypothesized that the observed ORR would be ∼70% based on assumptions derived from previous published experiences with frontline bortezomib combination regimens.5,7,10 A single-sided lower 95% confidence bound with a sample size of 30 and an ORR of 70% (ie, 21 responses of 30 patients) would equal 53.5% when the exact binomial method was used. If the obtained ORR single-sided lower 95% confidence interval (CI) was <40%, further study of the regimen in this indication would be deemed not warranted. Sixteen or fewer responders of 30 patients (ORR <56.7%) would result in a single-sided lower ORR CI of <40%.
Response determinations were made using consensus criteria adopted from the Sixth International Workshop on WM.22 CR was defined as resolution of all symptoms, normalization of serum IgM levels with complete disappearance of IgM paraprotein by immunofixation, absence of BM disease, and resolution of any adenopathy or splenomegaly. Patients with VGPRs, PRs, and MRs were defined as a ≥90% or normalization of serum IgM, ≥50%, and 25% to 49% reduction in serum IgM levels, respectively. Progressive disease was defined as a >25% increase (with an absolute 500 mg/dL) in serum IgM from nadir with reconfirmation required, in the absence of a suspected rituximab-induced flare, or progression of clinically significant disease-related symptoms.
Time to progression was defined as the time between initiation of therapy and date of progression, death, or last follow-up. For time to event analyses with censoring, the Kaplan-Meier method was used to estimate survival curves, which were compared using the log-rank test. The resulting hazard ratio (HR) was presented with the 95% CI. A Wilcoxon rank-sum test was used for analysis of pre- and posttherapy continuous variables. For categorical variables such as response vs nonresponse, a 2-tailed Fisher’s exact test was used. P < .05 was considered statistically significant. All graphics and calculations were obtained using STATA 13.1 (StataCorp LP, College Station, TX).
Results
Patients and disease characteristics
Thirty-one patients were enrolled, which included 1 patient above the intended study sample who consented simultaneously with subject 30 and was allowed to participate following Institutional Review Board approval. The baseline characteristics for these patients are shown in Table 1. The International Scoring System for WM (ISSWM) score for these patients was low (11; 35.5%); intermediate (15; 48.4%), and high (5; 16.1%).23 MYD88 and CXCR4 somatic mutation status was evaluable in 30 patients. Twenty-nine (96.6%) patients demonstrated the MYD88L265P mutation, and 11 (36.7%) had CXCR4WHIM mutations, including 7 with nonsense C-terminal truncating mutations and 4 with C-terminal frameshift mutations. Twenty-eight (90.3%) patients were previously untreated, and 3 patients received everolimus on a clinical protocol. Reasons (multiple causes can apply) for treatment initiation based on WM consensus criteria were symptomatic anemia (n = 30), extramedullary disease (n = 5), hyperviscosity (n = 4), and IgM-related PN (n = 3). The median number of treatment cycles administered including induction and maintenance was 12 (range, 4-14). Ten patients completed all 14 cycles of planned induction and maintenance therapy, and 8 patients are currently receiving maintenance therapy. Protocol therapy was truncated for cardiomyopathy (n = 1), progressive IgA/IgG hypogammaglobulinemia and recurring sinobronchial infections (n = 2), and nonresponse or progression (n = 10).
Baseline characteristics for all patients enrolled on study
| Characteristics . | Median . | Range . |
|---|---|---|
| Age (years) | 61 | 47-75 |
| Gender (M/F) | 19/12 | N/A |
| Serum IgM (mg/dL) | 3375 | 345-7430 |
| Serum IgA (mg/dL) | 47 | 18-725 |
| Serum IgG (mg/dL) | 715 | 222-3890 |
| Hemoglobin (g/dL) | 10.7 | 8.3-13.3 |
| Hematocrit (%) | 32.3 | 24.1-38.0 |
| Platelet (mm3) | 255 000 | 68 000-584 000 |
| B2-microglobulin (mg/L) | 3.6 | 1.9-7.2 |
| ISSWM score | 2 | 0-3 |
| Adenopathy | 18 (58.1%) | N/A |
| Splenomegaly | 2 (6.5%) | N/A |
| Bone marrow involvement (%) | 60 | 10-95 |
| Characteristics . | Median . | Range . |
|---|---|---|
| Age (years) | 61 | 47-75 |
| Gender (M/F) | 19/12 | N/A |
| Serum IgM (mg/dL) | 3375 | 345-7430 |
| Serum IgA (mg/dL) | 47 | 18-725 |
| Serum IgG (mg/dL) | 715 | 222-3890 |
| Hemoglobin (g/dL) | 10.7 | 8.3-13.3 |
| Hematocrit (%) | 32.3 | 24.1-38.0 |
| Platelet (mm3) | 255 000 | 68 000-584 000 |
| B2-microglobulin (mg/L) | 3.6 | 1.9-7.2 |
| ISSWM score | 2 | 0-3 |
| Adenopathy | 18 (58.1%) | N/A |
| Splenomegaly | 2 (6.5%) | N/A |
| Bone marrow involvement (%) | 60 | 10-95 |
N/A, not applicable.
Response
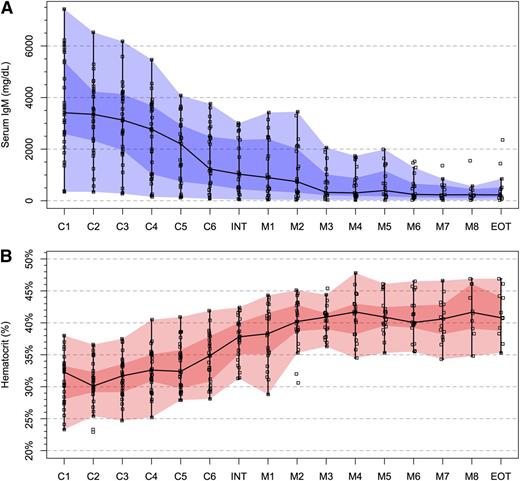
Median IgM levels for all 31 patients declined from 3375 (range, 345-7430 mg/dL) at baseline to 749 (range, 22-4540 mg/dL) at best response (P < .0001). Before therapy, 20 of 31 (64.5%) patients demonstrated a serum IgM level >3000 mg/dL; following treatment, 4 of 31 (12.9%) patients had a serum IgM level >3000 mg/dL (P < .0001). Median BM involvement decreased from 60% (range, 10-95%) to 5% (range, 0-95%) at best response (P < .0001), whereas the hematocrit rose from a median of 32.3% (range, 24.1-38.0%) to 41.3% (range, 30.9-47.8%) at best response (P < .0001). Serum IgM and hematocrit levels during the course of active therapy are shown in Figure 1. Categorical responses were as follows: CR (n = 1); VGPR (n = 10); PR (n = 10); and MR (n = 6), for an ORR and major response (PR or better) rate of 87.1% (95% CI, 75.3-98.9%) and 67.7% (95% CI, 51.2-84.2%), respectively. A CR/VGPR was achieved in 36% of patients. Overall (P = .633), major (P = .776), and VGPR/CR (P = .785) attainment was not impacted by ISSWM score. Nearly all (96.6%) genotyped patients expressed the MYD88L265P somatic mutation. The 1 patient with MYD88 wild type achieved a VGPR. CXCR4WHIM mutations were present in 11 of 30 (36.7%) study participants. The ORR for patients with CXCR4 wild-type and WHIM mutations were 85% and 90.9%, respectively (P = 1.0). Attainment of PR or better occurred in 65% and 73% of patients with CXCR4 wild-type and WHIM mutations, respectively (P = .711). No significant differences in ORR or major responses were observed between patients with nonsense or frameshift CXCR4WHIM mutations (data not shown). Among the 3 previously treated patients, each of whom received everolimus prior to study entry, 1 achieved a VGPR; 1 achieved a PR; and 1 was a nonresponder who subsequently responded to ibrutinib.
Impact of CaRD therapy on serum IgM and hematocrit levels. (A) Serum IgM and (B) hematocrit levels during the course of protocol therapy including induction cycles (C1-C6), interphase between induction and maintenance therapy (INT), maintenance cycles (M1-M8), and end of treatment (EOT) for all 31 patients who received CaRD. The black line and darker and lighter shaded areas represent the median, interquartile, and range of datapoints, respectively.
Impact of CaRD therapy on serum IgM and hematocrit levels. (A) Serum IgM and (B) hematocrit levels during the course of protocol therapy including induction cycles (C1-C6), interphase between induction and maintenance therapy (INT), maintenance cycles (M1-M8), and end of treatment (EOT) for all 31 patients who received CaRD. The black line and darker and lighter shaded areas represent the median, interquartile, and range of datapoints, respectively.
Among responders, the median time to at least a minor response was 2.1 (range, 0.7-14.7) months, whereas the median time to best response was 12.8+ (range, 2.1-25+) months. Among 18 patients with CT-defined adenopathy (>1.5 cm), adenopathy decreased or resolved (n = 18; 55.6%), remained stable (n = 5; 27.8%), or increased (n = 3; n = 16.8%) following therapy. Two patients with CT-defined splenomegaly (>15 cm) showed complete resolution after therapy. Three patients had IgM-related PN at baseline; 2 showed no change, and 1 had progression from grade 1 to grade 2 PN that prompted carfilzomib dose reduction to level −1; no further progression in PN then occurred.
Time to progression
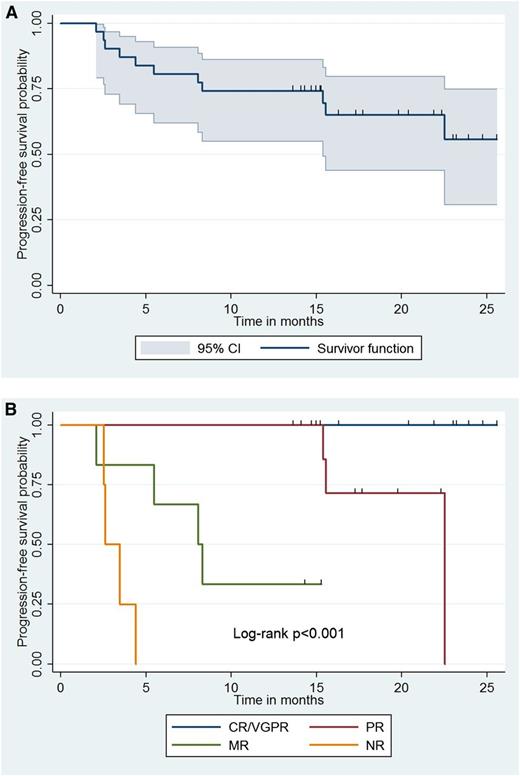
With a median follow-up of 15.4 (range, 2.1-25.5) months, all patients are alive, and 20 (64.5%) remain free of disease progression. The Kaplan-Meier curve for time to progression for all patients appears in Figure 2. Estimation for median time to progression was not possible due to insufficient progression events within the follow-up period. By univariate analysis, BM disease burden (HR, 1.03; 95% CI, 1.00-1.06; P = .034) and adenopathy (HR, 8.96; 95%, CI 1.14-70.3; P = .037) were associated with increased risk for progression. Categorical response attainment was also associated with risk of progression as shown in Figure 2; patients achieving an MR or PR exhibited shorter time to progression vs those with VGPR/CR (P < .001). There were no deaths during the follow-up period, and all patients are alive at this time.
Impact of CaRD therapy and response attainment on progression-free survival. Kaplan-Meier curve denoting (A) progression-free survival for all 31 patients and (B) based on categorical response following treatment with CaRD; 95% CI for survivor function is shown. NR, no response.
Impact of CaRD therapy and response attainment on progression-free survival. Kaplan-Meier curve denoting (A) progression-free survival for all 31 patients and (B) based on categorical response following treatment with CaRD; 95% CI for survivor function is shown. NR, no response.
Toxicities
Grade 1 to 4 toxicities that were at least possibly related to protocol therapy are presented in Table 2. The most common grade >2 toxicities included dexamethasone-related hyperglycemia (77.4%), carfilzomib-related hyperlipasemia (41.9%), and rituximab-related infusion reactions (19.4%). Hyperglycemia and hyperlipasemia were asymptomatic and reversible in all patients. Hyperlipasemia was often accompanied by hyperamylasemia and prompted treatment hold (n = 6) and/or dose reduction of carfilzomib (n = 5). Grade 2 rituximab reactions occurred in 6 patients and led to rituximab cessation in 3 patients after 2, 9, and 16 infusions. All 3 of these patients progressed during the follow-up period. Grade >2 neutropenia occurred in 3 (12.9%) patients and was reversible in all cases. Two patients (grade 3, 1; grade 4, 1) required treatment delay and filgrastim until neutrophil recovery. Both patients continued with protocol therapy after neutrophil recovery without any dose reductions or delays. One patient with grade 3 neutropenia had treatment delayed and recovery of their neutrophil count without filgrastim. Grade >3 neutropenia events were possibly related to either carfilzomib or rituximab. Anemia related to protocol therapy was rare. One patient who was a nonresponder progressed from baseline grade 2 to grade 3, and 2 patients progressed from grade 1 to grade 2 anemia (both responders), which subsequently normalized. Azotemia occurred in 2 patients (1 in cycle 2; 1 in cycle 5) and resolved after hydration. Grade 2 increases in bilirubin related to carfilzomib occurred in 2 patients and were asymptomatic. In 1 patient, famotidine may have contributed to hyperbilirubinemia and was discontinued without recurrence. Treatment-related grade 2 PN occurred in 1 patient with baseline grade 1 disease-related PN without pain and prompted reduction in carfilzomib to level −1. This patient’s neuropathy did not change following reduction of carfilzomib to level −1. Chest pain occurred in 1 patient, and extensive workup suggested a likely musculoskeletal etiology for this event. Cardiomyopathy triggered cessation of protocol therapy at cycle 4 in 1 patient. No baseline echocardiogram was available for this patient with heavy alcohol consumption and smoking (>60 pack-years) use. At the time of the event, echocardiogram showed 35% to 40% ejection fraction, and the patient was in atrial fibrillation. Extensive cardiological workup was unrevealing including for cardiac amyloidosis. The patient’s atrial fibrillation resolved after diuresis, and repeat echocardiogram showed stable ejection fraction. The patient subsequently discontinued alcohol and smoking consumption and remained asymptomatic without progression off-protocol therapy. A repeat echocardiogram obtained 6 months later showed a normal ejection fraction.
Adverse events possibly, probably, or definitely associated with protocol therapy
| Toxicity type . | Any grade . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Anemia | 3 (9.7%) | 0 (0%) | 2 (6.5%) | 1 (3.2%) | 0 (0%) |
| Arthralgia | 3 (9.7%) | 3 (9.7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Azotemia | 3 (9.7%) | 1 (3.2%) | 2 (6.5%) | 0 (0%) | 0 (0%) |
| Cardiomyopathy | 1 (3.2%) | 0 (0%) | 0 (0%) | 1 (3.2%) | 0 (0%) |
| Chest pain (non-cardiac) | 1 (3.2%) | 0 (0%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Dyspepsia | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fatigue | 2 (6.5%) | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Hyperglycemia | 31 (100%) | 7 (22.6%) | 17 (54.8%) | 7 (22.6%) | 0 (0%) |
| Hyperamylasemia | 8 (25.8%) | 7 (22.6%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Hyperbilirubinemia | 9 (29.0%) | 7 (22.6%) | 2 (6.5%) | 0 (0%) | 0 (0%) |
| Hyperkalemia | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperlipasemia | 17 (54.8%) | 4 (12.9%) | 8 (25.8%) | 5 (16.1%) | 0 (0%) |
| Hypokalemia | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Insomnia | 2 (6.5%) | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Infusion reaction (rituximab) | 7 (22.6%) | 1 (3.2%) | 6 (19.4%) | 0 (0%) | 0 (0%) |
| Mucositis | 2 (3.2%) | 2 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Neutropenia | 11 (34.8%) | 7 (22.6%) | 1 (3.2%) | 2 (6.5%) | 1 (3.2%) |
| Peripheral neuropathy | 6 (19.4%) | 5 (16.1%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Rash | 9 (29.0%) | 6 (19.4%) | 3 (9.7%) | 0 (0%) | 0 (0%) |
| Thrombocytopenia | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Toxicity type . | Any grade . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . |
|---|---|---|---|---|---|
| Anemia | 3 (9.7%) | 0 (0%) | 2 (6.5%) | 1 (3.2%) | 0 (0%) |
| Arthralgia | 3 (9.7%) | 3 (9.7%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Azotemia | 3 (9.7%) | 1 (3.2%) | 2 (6.5%) | 0 (0%) | 0 (0%) |
| Cardiomyopathy | 1 (3.2%) | 0 (0%) | 0 (0%) | 1 (3.2%) | 0 (0%) |
| Chest pain (non-cardiac) | 1 (3.2%) | 0 (0%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Dyspepsia | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Fatigue | 2 (6.5%) | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Hyperglycemia | 31 (100%) | 7 (22.6%) | 17 (54.8%) | 7 (22.6%) | 0 (0%) |
| Hyperamylasemia | 8 (25.8%) | 7 (22.6%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Hyperbilirubinemia | 9 (29.0%) | 7 (22.6%) | 2 (6.5%) | 0 (0%) | 0 (0%) |
| Hyperkalemia | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hyperlipasemia | 17 (54.8%) | 4 (12.9%) | 8 (25.8%) | 5 (16.1%) | 0 (0%) |
| Hypokalemia | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Insomnia | 2 (6.5%) | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Infusion reaction (rituximab) | 7 (22.6%) | 1 (3.2%) | 6 (19.4%) | 0 (0%) | 0 (0%) |
| Mucositis | 2 (3.2%) | 2 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Neutropenia | 11 (34.8%) | 7 (22.6%) | 1 (3.2%) | 2 (6.5%) | 1 (3.2%) |
| Peripheral neuropathy | 6 (19.4%) | 5 (16.1%) | 1 (3.2%) | 0 (0%) | 0 (0%) |
| Rash | 9 (29.0%) | 6 (19.4%) | 3 (9.7%) | 0 (0%) | 0 (0%) |
| Thrombocytopenia | 1 (3.2%) | 1 (3.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
Numbers given as number (percentage) of events.
Rituximab-induced IgM flare
Nine patients underwent prophylactic pretherapy plasmapheresis, which included 4 patients for whom omission of rituximab occurred for ≥1 cycle. Of the 22 patients who did not require prophylactic plasmapheresis, a flare in serum IgM level (≥25%) associated with rituximab was observed in 5 (22.7%) patients and prompted plasmapheresis in 1 patient.
IgA and IgG hypogammaglobulinemia
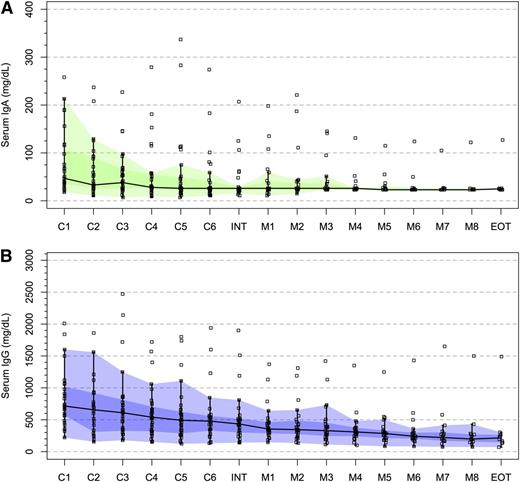
At baseline, median serum IgA and IgG levels were 47 and 715 mg/dL, respectively, with 21 (67.7%) and 15 (48.4%) patients demonstrating IgA and IgG hypogammaglobulinemia. Following therapy, median serum IgA and IgG levels declined to 23 and 238 mg/dL (P = .002 and P < .0001, respectively), with 28 (90.3%) and 28 (90.3%) patients demonstrating IgA and IgG hypogammaglobulinemia (P = .058 and .0003, respectively). Serum IgA and IgG levels for all patients during the course of therapy are shown in Figure 3. Protocol therapy was truncated after maintenance cycle 7 in 2 patients with recurring sinobronchial infections associated with treatment-aggravated IgG hypogammaglobulinemia, and for whom intravenous gammaglobulin therapy was initiated. Two other patients required intravenous gammaglobulin for treatment-aggravated IgG hypogammaglobulinemia and recurring sinobronchial infections but continued on protocol therapy.
Impact of CaRD therapy on serum IgA and IgG levels. (A) Serum IgA and (B) IgG levels during the course of protocol therapy including induction cycles (C1-C6), interphase between induction and maintenance therapy (INT), maintenance cycles (M1-M8), and end of treatment (EOT) for all 31 patients who received CaRD. The black line and darker and lighter shaded areas represent the median, interquartile, and range, respectively.
Impact of CaRD therapy on serum IgA and IgG levels. (A) Serum IgA and (B) IgG levels during the course of protocol therapy including induction cycles (C1-C6), interphase between induction and maintenance therapy (INT), maintenance cycles (M1-M8), and end of treatment (EOT) for all 31 patients who received CaRD. The black line and darker and lighter shaded areas represent the median, interquartile, and range, respectively.
Discussion
Treatment-related PN related to bortezomib is common and often can be severe and debilitating in WM patients.12 Carfilzomib is a neuropathy-sparing second-generation proteasome inhibitor.13 We therefore examined CaRD as a frontline treatment option for WM patients and observed an ORR of 87.1%. Importantly, 36% of patients achieved a VGPR/CR, including 1 patient who attained a molecular CR. At baseline, this patient had strongly detectable MYD88L265P-positive BM and PB disease with ΔCTs of 1.8 and 3.8, respectively, using a highly sensitive allele-specific-PCR assay.24,25 Following maintenance cycle 3 (to confirm CR status) and maintenance cycle 8 (end of therapy visit), MYD88L265P was undetectable in both BM and PB samples using the same allele-specific-PCR assay, thereby constituting the first observation of molecular CR attainment in WM.
Both MYD88L265 and CXCR4WHIM mutation statuses were also evaluated to clarify impact on response attainment. Nearly all patients (96.6%) who were genotyped expressed MYD88L265P, precluding a meaningful analysis, although it is interesting that the single patient who was wild-type MYD88 demonstrated a VGPR. No significant differences in either overall or major responses were observed between patients with CXCR4 wild-type or WHIM mutations who received CaRD. The presence of CXCRWHIM somatic mutations was a key determinant for response in a study of relapsed/refractory patients receiving ibrutinib, wherein lower response rates were observed in CXCR4WHIM-expressing patients.18 These findings, although confined to frontline patients, suggest that the activity of CaRD is independent of CXCR4WHIM mutation status and can be observed even with MYD88 wild-type mutation status.
The clinical responses achieved with CaRD were comparable with those achieved using bortezomib in the bortezomib, dexamethasone, rituximab (BDR) regimen wherein an ORR of 88% to 95% and a VGPR of 10% to 35% were observed in frontline patients.5,10 The attainment of VGPR/CR is of particular interest given the association of longer PFS in WM patients receiving a rituximab-containing regimen, including proteasome inhibitor therapy.5,9,10 Consistent with this finding, patients on this study who attained at least a VGPR during the follow-up period demonstrated longer PFS vs patients who attained a minor or partial response. With a median follow-up of 15.4 months, 64% of patients remained free of disease progression, and a longer follow-up will invariably be required to establish the relative PFS for CaRD vs BDR, in which PFS of 42 months to >4 years have been reported.5,10
Another important consideration was the relative rapid response time with CaRD. The median time to a ≥25% reduction in serum IgM with CaRD was 2.1 months, which is similar to that reported with BDR (1.5-3 months) and compares favorably against other commonly used therapies for WM with response times of >3 to 4 months.21,26,27 In WM patients in whom more rapid responses may be required, eg, patients with symptomatic hyperviscosity, cryoglobulinemia, autoimmune cytopenias, etc, CaRD may represent an attractive treatment option.
Overall, treatment with CaRD was well tolerated. Asymptomatic increases in lipase, amylase, and bilirubin were common, occurred in 25% to 50% of patients, and were associated with carfilzomib and dexamethasone administration. The incidence for these events is much higher than reported with other carfilzomib studies, as well as for WM-related studies with bortezomib, dexamethasone, and/or rituximab.5-8,10,13 These events recurred for many patients after treatment with carfilzomib and dexamethasone and typically resolved by the subsequent cycle. No baseline clinical or disease features correlated with these anomalies, and in 1 patient, cessation of famotidine led to resolution of recurring hyperbilirubinemia. Reversible grade 2 azotemia occurred in 2 patients that resolved after hydration. Overall, hematological toxicities were unimpressive, with absent or modest levels of grade 3 or higher anemia (3.2%), neutropenia (6.5%), and thrombocytopenia (0%). By comparison, grade >3 neutropenia (12-15%) and thrombocytopenia (5-8%) were more common, whereas levels of grade >3 anemia were similar (0-8%) in WM patients receiving frontline bortezomib combination therapy.5,7,10
Central to this study’s findings was the paucity of treatment-related PN. Only 1 patient (3.2%) experienced grade 2 treatment-related PN, and no patient had discontinuation of CaRD for PN. By comparison, grade 2 or higher PN occurred in 20% to 70% of WM patients who received frontline bortezomib-based therapy, with discontinuation of bortezomib in 10% to 60% of cases.4,5,7,10 In many of these cases, bortezomib-related PN continued long after discontinuation of therapy and necessitated in many patients chronic gabapentin, pregabalin, and/or narcotic use.11 The high incidence of treatment-related PN also limits prolonged or maintenance use of bortezomib for many patients, thereby limiting the intended therapeutic effects.
Cardiomyopathy occurred in 1 patient (3.2%), which resulted in premature cessation of intended therapy at induction cycle 4. Cardiomyopathy has been rarely reported in other studies with carfilzomib, with an estimated incidence of 7% in a large meta-analysis of 4 studies in relapsed/refractory myeloma patients.13 Previous exposure to cardiotoxic chemotherapy, including high-dose chemotherapy, could have contributed to these events, and a class effect for proteasome inhibitors might also be relevant because cardiotoxicity has been reported with bortezomib.28,29 Smoking, heavy alcohol use, and tachyarrhythmia may also have contributed to the single cardiomyopathy event in this study. This patient fully recovered following discontinuation of protocol therapy along with smoking, alcohol consumption, and resolution of tachyarrhythmia. Taken together, these findings necessitate continued pharmaco-vigilance until the relative risk of cardiomyopathy associated with carfilzomib is better understood. Ongoing studies examining bortezomib vs carfilzomib-based therapy in newly diagnosed myeloma patients will be particularly illuminating and may provide insights into patients at risk for cardiomyopathy. In the interim, in WM patients who have risk factors for cardiomyopathy, the use of carfilzomib can be considered against other options, and baseline and periodic follow-up echocardiography should be considered.
IgA and IgG hypogammaglobulinemia are common in WM patients and can be aggravated with rituximab-containing induction and maintenance therapy.30,31 At baseline, most patients in this study exhibited IgA and IgG hypogammaglobulinemia, in line with our previous observations.30 Following CaRD therapy, IgA and IgG levels declined to 48% and 33% of baseline levels and resulted in treatment truncation at maintenance cycle 7 for 2 patients who developed recurring sinobronchial infections requiring antibiotic therapy. Close monitoring of IgA and IgG levels should therefore be undertaken in WM patients undergoing CARD therapy, and ongoing maintenance therapy given in the context of changes to IgA and IgG levels and infection risk. Studies examining alternative (ie, maintenance every 3 months) or truncated (ie, 6 vs 8 cycles) maintenance schedules should also be considered in future studies to minimize IgA and IgG depletion with CaRD.
In summary, CaRD produces high rates of response including VGPR or better in symptomatic frontline patients with WM. Responses are rapid, and treatment is well tolerated, with minimal treatment-related neuropathy. Patients at risk for cardiomyopathy should be carefully monitored or alternative treatment options should be considered. Asymptomatic elevations in lipase, amylase, and bilirubin levels are common with CaRD, whereas IgA and IgG depletion occurs in most patients and can be associated with recurring sinobronchial infections. CaRD offers a neuropathy-sparing approach for proteasome inhibitor-based therapy in WM.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Peter and Helen Bing Fund for Waldenström’s Macroglobulinemia at the Dana Farber Cancer Institute, the Linda and Edward Nelson Fund, and Onyx Pharmaceuticals, who provided study support and carfilzomib for these studies.
Authorship
Contribution: S.P.T. designed the study; S.P.T., P.S., S.K., I.M.G., S.C., and J.J.C. provided treatment and follow-up for patients on the study; C.K.T., D.W., and K.M. coordinated the study and provided regulatory oversight; C.K.T., K.M., and C.J.P. collected the study data; L.X., G.Y., Y.C., X.L., and Z.R.H. performed genotyping for MYD88L265P and CXCR4WHIM mutations; S.P.T., K.M., C.K.T., Z.R.H., B.T., and J.J.C. analyzed the study data; and S.P.T. wrote the manuscript.
Conflict-of-interest disclosure: S.P.T. and I.M.G. have received research funding, speaking honoraria, and/or consulting fees from Onyx Pharmaceuticals. All other authors declare no competing financial interests.
Correspondence: Steven P. Treon, Bing Center for Waldenström’s Macroglobulinemia, Dana-Farber Cancer Institute, Harvard Medical School, M548, 450 Brookline Ave, Boston, MA 02215; e-mail: steven_treon@dfci.harvard.edu.