In this issue of Blood, Chitteti et al show that CD166 is a potential marker for both human and murine hematopoietic stem cells (HSCs) and that the osteoblastic niche plays a critical role in the maintenance of HSCs through homotypic interactions of CD166.1
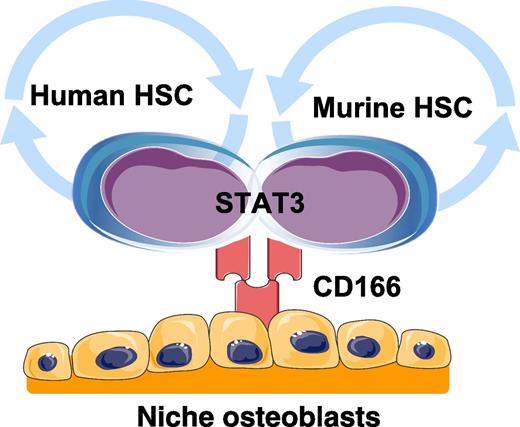
Human and murine HSCs interact with niche osteoblasts through homotypic interactions with CD166. Engagement of CD166 by HSCs activates STAT3 signaling. Graphics adapted from Servier Medical Art (www.servier.com).
Human and murine HSCs interact with niche osteoblasts through homotypic interactions with CD166. Engagement of CD166 by HSCs activates STAT3 signaling. Graphics adapted from Servier Medical Art (www.servier.com).
The identity of HSCs is an active area of investigation. Chitteti et al report that activated leukocyte cell adhesion molecule (CD166) regulates HSC-niche osteoblast interactions through homotypic interactions, but also identifies both human and murine long-term repopulating cells (see figure). Accumulating evidence suggests that HSCs reside in marrow in a safe harbor that protects and maintains stem cells in a quiescent or G0 state. This hematopoietic microenvironment, or niche, regulates HSC function and activation through many soluble and adhesive interactions. Defining those cell-extrinsic factors that regulate HSC fate, differentiation, and self-renewal is critical to our knowledge of normal stem cell biology and is clinically relevant in both transplantation and the treatment of liquid and solid tumors.
To determine whether CD166 identifies murine HSCs, Chitteti et al first performed transplantation assays into lethally irradiated mice with four different candidate phenotypes: Lin−cKit+Sca1+(LSK)CD48− cells, CD166+CD150−LSKCD48− cells, CD166−CD150+LSKCD48− cells, or CD166+CD150+LSKCD48− cells. Cells expressing the CD166+CD150+LSKCD48− phenotype engrafted significantly better than the other cell types and tended to differentiate into myeloid lineages. Limiting dilution assays (frequency of HSCs: 1/17) and serial transplantation assays (levels of chimerism: 20-fold higher than other cell types) confirmed these observations. In addition, approximately 30% of CD9+CD150+LSKCD48− cells2 and the side population (phenotypes with punitive stem cell activities) express CD166. Moreover, only CD166+CD9+CD150+LSKCD48− cells possessed engraftment ability, whereas CD166−CD9+CD150+LSKCD48− cells did not.
Using human cord blood, the relevance of CD166 expression on human HSCs was next determined. As in the mouse model, well-studied human HSCs (Lin−CD34+CD38− cells) were divided based on CD166 expression, and xenograft transplantation was performed into sublethally irradiated NSG mice. CD166+Lin−CD34+CD38− cells showed significantly better engraftment than CD166−Lin−CD34+CD38− cells. To further confirm the potential of CD166 as a human HSC marker, cord blood was segregated on the basis of CD166 and CD49f. Independent of CD49f expression, CD166-expressing Lin−CD34+CD38− cells showed better engraftment even in the serial transplantation assays.
To further confirm these findings, similar experiments were repeated using CD166-deficient (CD166−/−) mice. Both LSKCD48− cells and CD150+LSKCD48− cells were significantly reduced in the CD166−/− mice, and these mice had more lymphoid cells. Additionally, colony-forming ability of hematopoietic progenitor cells were significantly reduced in these animals, although the response of mobilization mediated by granulocyte colony-stimulating factor was not altered. The engraftment of LSK cells isolated from CD166−/− mice was impaired and provided less radioprotection than cells isolated from CD166+/+ mice. Critically, when CD166+/+ LSK cells were transplanted into CD166−/− mice, hematopoietic reconstitution was compromised. These findings were confirmed using in vitro adhesion assays and colony formation assays with coculture combination of LSK cells and osteoblasts obtained from both CD166+/+ and CD166−/− mice. Surprisingly, no differences in adhesion molecules known to regulate HSC–osteoblast interactions were noted comparing CD166+/+ and CD166−/− HSCs (CD44, CD49d, CD49e, CD61, CD62L, CD144, CD162, or CXCR4). It was also noted that CD166−/− mice were less tolerant to cytotoxic insults (5-fluorouracil and radiation), and CD166−/− LSK cells were less frequently in G0, having progressed into G1/S/G2/M relative to controls.
Interestingly, CD166 did not segregate the ability of HSCs to home to the marrow of either CD166+/+ or CD166−/− mice. However, when HSCs isolated from CD166+/+ or CD166−/− mice were transplanted into CD166+/+ or CD166−/− mice, the distances between the transplanted cells and the endosteal surfaces were significantly greater compared with those distances established by CD166+/+ HSCs.
To further examine the role that CD166 has in the bone marrow niche, osteoblasts from CD166+/+ and CD166−/− mice were examined. There were no differences in the osteoblastic phenotype of the mice; however, HoxB4 and N-cadherin expression was lower in the CD166−/− osteoblasts, but not SDF-1. Chromatin immunoprecipitation identified CD166 and signal transducer and activator of transcription 3 (STAT3) interactions possibly involving Survivin, a transcriptional target of STAT3. HSCs (LSK cells and CD150+ LSK cells) obtained from the STAT3−/− mice expressed significantly less CD166 compared to those obtained from STAT3+/+ mice. When bone marrow progenitor cells from STAT3+/+ mice were treated with a STAT3 inhibitor, CD166 expression was reduced. Moreover, when CD166 expression in MOLT4 cells was downregulated by short hairpin RNA, STAT3 was significantly downregulated.
There are several significant findings reported by Chitteti et al.1 First, the identification and regulatory circuits controlling HSC function by the HSC niche has been hotly debated. The importance of a marrow niche organ for regulating HSC activities was first suggested by Schofield in 1978.3 Osteoblasts first appeared as candidates for niche function in 1994 when Taichman et al demonstrated in vitro that human osteoblasts secrete granulocyte colony-stimulating factor and are able to maintain human long-term culture initiating cells.4,5 In 2003, consecutive reports by Scadden’s and Le’s groups using murine systems demonstrated that perturbations of osteoblast function regulates HSC numbers and activities.6,7 Which specific osteoblastic lineage subpopulations serve as niche partners with HSCs remains under investigation, as does the participation of other niche constituents including endothelial cells, adipocytes, nerves, and CXCL12-associated reticular cells.8 Yet accumulating evidence also suggests that niche osteoblasts may participate in the development of leukemic disease in which alterations in microRNA processing and β-catenin signaling in cells of the osteoblastic lineage result in not only osseous defects but also leukemia.9,10 The findings show that interactions between HSCs and cells of the osteoblast lineage based on CD166 expression provide further clarity that multiple cell types participate in niche function. Second, and perhaps most critically, CD166 expression marks both human and murine HSCs. These findings will likely simplify the isolation of HSCs and enhance translation of results made in mouse into humans by identifying antigens conserved by HSCs of both species.
Conflict-of-interest disclosure: The authors declare no competing financial interests.