Key Points
Severe thrombocytopenia is associated with a strongly impaired host defense during pneumonia-derived Klebsiella pneumoniae sepsis.
Platelet counts between 5 and 13 × 109/L of normal prevent bleeding and confer protection against distant organ damage during gram-negative sepsis.
Abstract
Thrombocytopenia is a common finding in sepsis and associated with a worse outcome. We used a mouse model of pneumonia-derived sepsis caused by the human pathogen Klebsiella pneumoniae to study the role of platelets in host response to sepsis. Platelet counts (PCs) were reduced to less than a median of 5 × 109/L or to 5 to 13 × 109/L by administration of a depleting antibody in mice infected with Klebsiella via the airways. Thrombocytopenia was associated with strongly impaired survival during pneumonia-derived sepsis proportional to the extent of platelet depletion. Thrombocytopenic mice demonstrated PC-dependent enhanced bacterial growth in lungs, blood, and distant organs. Severe thrombocytopenia resulted in hemorrhage at the primary site of infection, but not in distant organs. PCs of 5 to 13 × 109/L were sufficient to largely maintain hemostasis in infected lungs. Thrombocytopenia did not influence lung inflammation or neutrophil recruitment and did not attenuate local or systemic activation of coagulation or the vascular endothelium. PCs <5 × 109/L even resulted in enhanced coagulation and endothelial cell activation, which coincided with increased proinflammatory cytokine levels. In accordance, low PCs in whole blood enhanced Klebsiella-induced cytokine release in vitro. These data suggest that platelets play an important role in host defense to Klebsiella pneumosepsis.
Introduction
Sepsis is one of the most elusive syndromes in medicine, with an estimated incidence of over 19 million cases per year worldwide1,2 and high mortality rates despite appropriate antibiotic treatment.2 The most common cause of sepsis is pneumonia.3 Klebsiella pneumoniae is a frequent causative agent in gram-negative sepsis and a common respiratory pathogen.2,4,5
Thrombocytopenia is a common finding in patients admitted to the intensive care unit and is associated with a worse outcome.6-9 Platelets are small anucleate cells widely renowned for their role in hemostasis. Notably, however, low platelet counts (PCs) result in hemorrhage in only some patients. In accordance, thrombocytopenia per se does not induce bleeding in mice; however, platelets have a crucial role in preventing hemorrhage in inflamed organs.10 In addition to hemostasis, platelets contribute to host defense against bacteria and may mediate a variety of proinflammatory effects.11,12 On the other hand, platelets may inhibit macrophage-dependent inflammation during infectious and noninfectious systemic inflammation.13,14 These properties implicate platelets as essential players in the pathogenesis of sepsis, involved in both protective and potentially injurious host responses.15
In this study, we sought to determine the impact of thrombocytopenia on host defense during pneumonia-derived sepsis caused by Klebsiella. We administered a platelet-depleting antibody to mice at two different doses, which caused thrombocytopenia of different severities, and studied bacterial growth and dissemination, hemostasis, and local and systemic inflammation after infection with a virulent K pneumoniae strain via the airways.
Methods
Animals
Female C57BL/6 mice (Harlan Laboratories, Horst, The Netherlands) between 10 and 12 weeks of age were used. The Institutional Animal Care and Use Committee of the Academic Medical Center approved all experiments.
Experimental study design
Pneumonia was induced by intranasal inoculation with K pneumoniae serotype 2 (43816; American Type Culture Collection, Rockville, MD); 104 colony-forming units (CFUs) in 50 μL isotonic saline) as described previously.16,17 Two hours before infection, mice were intravenously infused with 2 μg/g or 0.4 μg/g anti(α)-glycoprotein(Gp)Ibα (Emfret Analytics, Eibelstadt, Germany) or 2 μg/g control immunoglobulin (Ig)G (Emfret Analytics).10,18 Mice were euthanized 12 or 44 hours after induction of pneumonia (n = 8 mice per group at each time point) or observed for 7 days (n = 20 per group). During the observation study, clinical signs were scored by an independent animal biotechnician as described in the supplemental Methods on the Blood Web site. Lung, spleen, and liver were harvested, individually weighed, and homogenized in sterile isotonic saline (4 mL per gram of tissue). Bacterial quantification and storage of organs were performed as described previously.16,17
Flow cytometry
PCs were determined in citrated whole blood by flow cytometry (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ) using hamster anti-CD61 monoclonal antibody (BioLegend, San Diego, CA) in accordance with manufacturers’ instructions.
Histology and assays
Please see the supplemental Methods.
In vitro experiments
Mice were intravenously infused with 2 μg/g α-GpIbα or 2 μg/g control IgG, and heparinized blood was collected from the inferior vena cava 2 hours thereafter. Ninety microliters of whole blood was incubated with an equal volume of vehicle medium (Iscove modified Dulbecco medium [IMDM]; Lonza, Basel, Switzerland) with or without 105 CFUs of K pneumoniae. After 6 hours, part of the blood was serially diluted in normal saline, and CFUs were counted after 16 hours of incubation at 37°C. The remaining blood was spun at 3000 rpm for 10 minutes, and plasma supernatants were stored at −20°C until analysis.
Statistical analysis
Data are expressed as scatter dot plots or as box-and-whisker plots. Comparisons between groups were first performed using Kruskal-Wallis 1-way analysis of variance test, followed by the Mann-Whitney U test where appropriate. Survival was compared using the Kaplan-Meier method, followed by the log-rank test. To compare clinical observation scores, areas under the curve were compared using analysis of variance with rank transformation. P values <.05 were considered statistically significant.
Results
Infusion of α-GpIbα induces dose-dependent platelet depletion
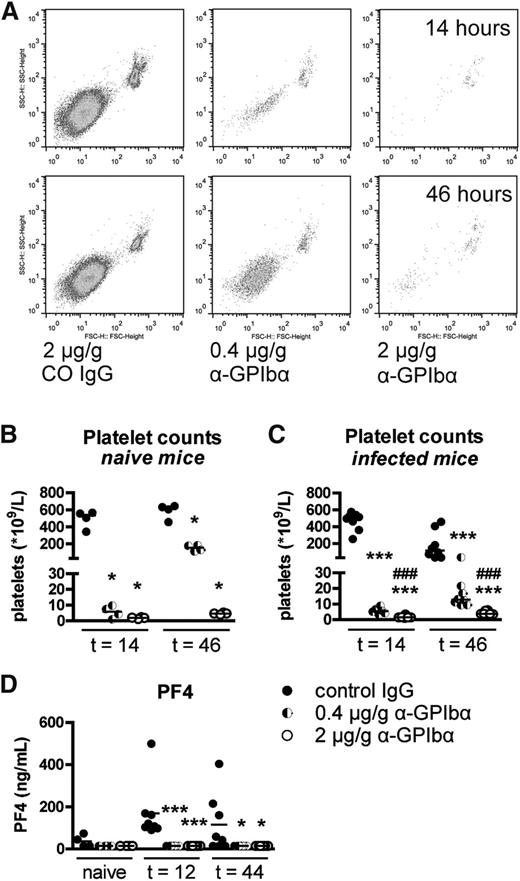
Thrombocytopenia was induced by injection of α-GpIbα antibodies, which deplete platelets in mice without affecting the function of the remaining platelet population.18 Naive mice had a median PC of 521 × 109/L. Infusion with 2 μg/g and 0.4 μg/g α-GpIbα caused platelet depletion to a median of 2 × 109 platelets/L and 6 × 109/L respectively, after 14 hours; PCs rose to a median of 5 × 109/L and 153 × 109/L, respectively, after 46 hours in naive mice (Figure 1A-B). Twelve hours after infection with Klebsiella (14 hours after α-GpIbα infusion), the extent of α-GpIbα-induced platelet depletion was similar compared with naive mice. After 44 hours of infection, however, PCs in control mice lowered to a median of 117 × 109 platelets/L in uninfected control mice. Whereas mice treated with 2 µg/g α-GpIbα still displayed PCs <5 × 109/L, mice infused with 0.4 μg/g α-GpIbα replenished platelets only to 13 × 109 platelets/L (Figure 1C). To confirm that platelet depletion by α-GpIbα does not cause platelet lysis or activation, plasma platelet factor (PF)4 was measured (Figure 1D). Although PF4 was upregulated during Klebsiella pneumosepsis in control mice (indicative of platelet activation during sepsis), no PF4 could be detected in α-GpIbα-treated mice at any time point. α-GpIbα did not induce alterations in any of the inflammatory outcome measurements in uninfected mice. Mice treated with 0.4 µg/g or 2 µg/g α-GpIbα are subsequently referred to as low-PC mice or very low-PC mice, respectively.
Infusion of α-GpIbα induces dose-dependent platelet depletion. PCs were determined in citrated whole blood by flow cytometry. (A) Log scale scatter plots of CD61-positive events in naive mice infused with 2 μg/g α-GpIbα, 0.4 μg/g α-GpIbα, or control IgG. PCs depicted as scatter dot plots with the median 14 and 46 hours after IV administration of 2 μg/g (open dots), 0.4 μg/g α-GpIbα (half-open dots), or control IgG (closed dots) in naive mice (B) and in Klebsiella-infected mice (C). (D) PF4 was measured in naive or in infected control and platelet-depleted mouse plasma. Data are expressed as scatter dot plots with the median. n = 4 (naive) mice and n = 8 (infected) mice per group. *P < .05 and ***P < .0005 vs control IgG; ###P < .0005 vs 0.4 μg/g α-GpIbα.
Infusion of α-GpIbα induces dose-dependent platelet depletion. PCs were determined in citrated whole blood by flow cytometry. (A) Log scale scatter plots of CD61-positive events in naive mice infused with 2 μg/g α-GpIbα, 0.4 μg/g α-GpIbα, or control IgG. PCs depicted as scatter dot plots with the median 14 and 46 hours after IV administration of 2 μg/g (open dots), 0.4 μg/g α-GpIbα (half-open dots), or control IgG (closed dots) in naive mice (B) and in Klebsiella-infected mice (C). (D) PF4 was measured in naive or in infected control and platelet-depleted mouse plasma. Data are expressed as scatter dot plots with the median. n = 4 (naive) mice and n = 8 (infected) mice per group. *P < .05 and ***P < .0005 vs control IgG; ###P < .0005 vs 0.4 μg/g α-GpIbα.
Thrombocytopenia is associated with a PC-dependent impaired survival in Klebsiella-induced pneumonia
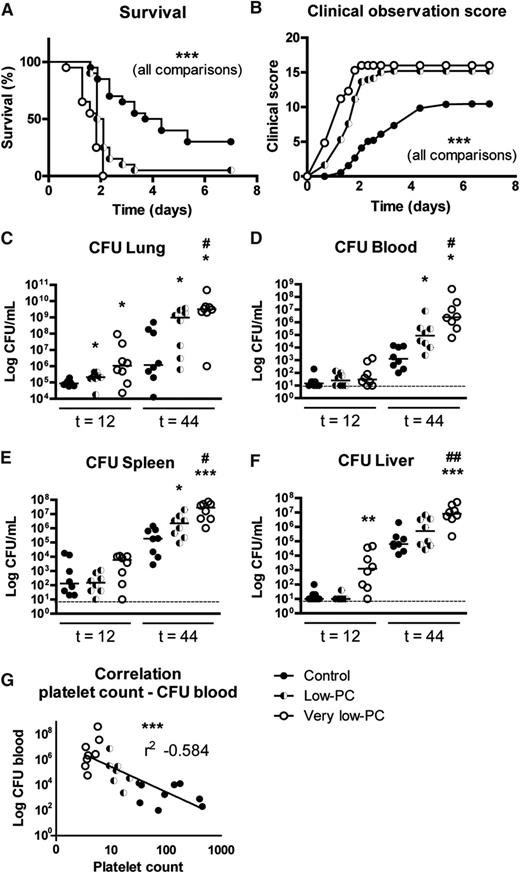
To determine the impact of thrombocytopenia on mortality during K pneumoniae-induced pneumonia, we carried out an observational study in very low-PC, low-PC, and control mice during 7 days after infection (Figure 2A-B). Very low-PC mice demonstrated a rapidly increasing symptom score shortly after infection and all succumbed in the second night (P < .0005 vs low-PC and control mice). Low-PC mice developed symptoms shortly after very low-PC mice and showed a relatively delayed mortality (P < .0005 vs control and very low-PC mice). Symptoms developed much slower in control mice, of which 30% survived the infection, whereas none of the very low-PC and only 1 of the low-PC mice survived.
Thrombocytopenia causes PC-dependent impaired survival and enhanced bacterial growth during Klebsiella-induced pneumonia. Survival (A) and clinical observation score (B) of very low-PC (open dots), low-PC (half-open dots), and control (closed dots) mice infected with K pneumoniae via the airways. For bacterial quantification, very low-PC (open dots), low-PC (half-open dots), and control (closed dots) mice were again infected with K pneumoniae via the airways and euthanized at the indicated time points. Bacterial counts were determined in lungs (C), blood (D), spleen (E), and liver (F). (G) Correlation of platelet counts and Klebsiella CFU recovered from blood 44 hours after infection. Data are expressed as scatter dot plots with the median. n = 20 mice per group in the survival experiment and n = 8 mice per group for bacterial quantification. ***P < .0005 for all comparisons in survival and clinical observation score graphs; *P < .05, **P < .005, and ***P < .0005 vs control; #P < .05 and ##P < .005 vs low-PC.
Thrombocytopenia causes PC-dependent impaired survival and enhanced bacterial growth during Klebsiella-induced pneumonia. Survival (A) and clinical observation score (B) of very low-PC (open dots), low-PC (half-open dots), and control (closed dots) mice infected with K pneumoniae via the airways. For bacterial quantification, very low-PC (open dots), low-PC (half-open dots), and control (closed dots) mice were again infected with K pneumoniae via the airways and euthanized at the indicated time points. Bacterial counts were determined in lungs (C), blood (D), spleen (E), and liver (F). (G) Correlation of platelet counts and Klebsiella CFU recovered from blood 44 hours after infection. Data are expressed as scatter dot plots with the median. n = 20 mice per group in the survival experiment and n = 8 mice per group for bacterial quantification. ***P < .0005 for all comparisons in survival and clinical observation score graphs; *P < .05, **P < .005, and ***P < .0005 vs control; #P < .05 and ##P < .005 vs low-PC.
Thrombocytopenia results in PC-dependent enhanced bacterial growth and dissemination
To obtain insight into the underlying mechanism by which thrombocytopenia impairs outcome in Klebsiella pneumosepsis, we determined bacterial loads in lungs, blood, spleen, and liver at 12 and 44 hours after infection. At 12 hours after infection, low-PC mice displayed a slightly enhanced bacterial outgrowth in the lungs (P < .05 vs control mice); very low-PC mice displayed more pronounced increased bacterial burdens in lungs and liver (P < .05 vs control mice; Figure 2C,F). These differences increased after 44 hours of infection (Figure 2C-F), with median bacterial loads that were ≥100-fold higher in all organs of very low-PC mice (P < .005 to P < .0005 vs control mice), and ≥10-fold higher in lungs, blood, and spleen of low-PC mice (P < .05 to P < .005 vs control mice). Notably, at this late time point, bacterial burdens were significantly higher in very low-PC mice relative to low-PC mice in all body sites tested (P < .05 to P < .005). Blood PCs negatively correlated with blood Klebsiella CFUs at 44 hours (Figure 2G).
Thrombocytopenia results in PC-dependent lung hemorrhage after infection, with intact hemostasis in distant organs
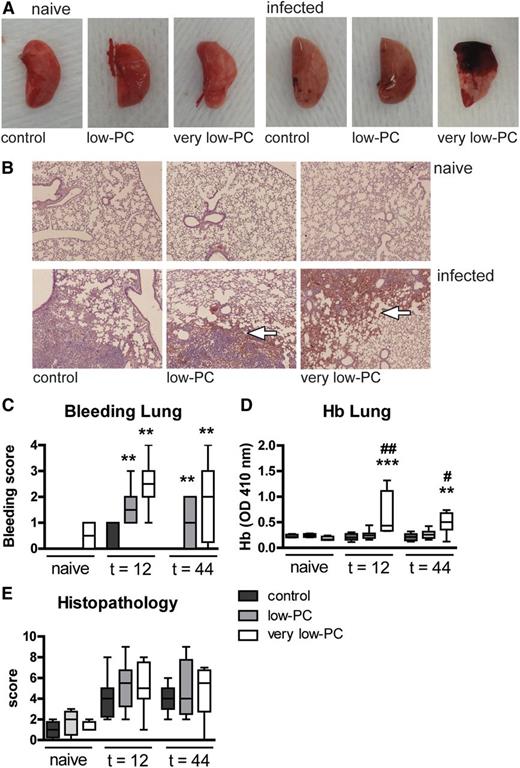
We examined the occurrence of hemorrhages at the primary site of infection (lungs) and two distant body sites (spleen and liver) in very low-PC, low-PC, and control mice in the presence or absence of Klebsiella pneumosepsis (Figure 3). Very low-PC mice demonstrated massive bleeding in their lungs after infection with K pneumoniae, both macroscopically (Figure 3A) and microscopically (Figure 3B-C). Whereas infected low-PC mice did not display macroscopic signs of lung bleeding (Figure 3A), microscopic examination of hematoxylin and eosin (H&E)-stained lung tissue sections revealed sites of hemorrhage (Figure 3B-C; P < .005 vs control mice at both 12 and 44 hours), albeit to a lesser extent than in very low-PC mice (P < .005 vs control mice and P = .06 for the difference between low-PC and very low-PC mice at 12 hours). Of interest, neither low-PC nor very low-PC mice showed hemorrhages in spleen or liver (supplemental Figure 1A-B). Bleeding was not found in any organ of uninfected mice from any of the three experimental groups. In accordance with histology, the infected lungs of very low-PC mice contained higher hemoglobin (Hb) levels relative to low-PC and control mice (P < .005 and P < .0005, respectively; Figure 3D), whereas in distant organs, Hb concentrations were similar in all groups (supplemental Figure 1C-D). The Hb content of organs from uninfected mice was also similar in all groups.
Thrombocytopenia results in PC-dependent lung hemorrhage after infection, with intact hemostasis in distant organs. Very low-PC, low-PC, and control mice were infected with K pneumoniae via the airways and euthanized at 12 or 44 hours. (A) Representative photographs of naive or infected lungs. (B) Representative photomicrographs of H&E-stained tissue sections of naive or infected lungs at 44 hours (original magnification ×4). (C) Lung bleeding was scored on H&E tissue sections by a pathologist blinded for groups. (D) Hb was measured in 50-fold diluted lung homogenates by light density at 410 nm. (E) Severity of inflammation was scored on H&E tissue sections. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation. n = 8 mice per group. **P < .005 and ***P < .0005 vs control; #P < .05 and ##P < .005 vs low-PC. OD, optical density.
Thrombocytopenia results in PC-dependent lung hemorrhage after infection, with intact hemostasis in distant organs. Very low-PC, low-PC, and control mice were infected with K pneumoniae via the airways and euthanized at 12 or 44 hours. (A) Representative photographs of naive or infected lungs. (B) Representative photomicrographs of H&E-stained tissue sections of naive or infected lungs at 44 hours (original magnification ×4). (C) Lung bleeding was scored on H&E tissue sections by a pathologist blinded for groups. (D) Hb was measured in 50-fold diluted lung homogenates by light density at 410 nm. (E) Severity of inflammation was scored on H&E tissue sections. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation. n = 8 mice per group. **P < .005 and ***P < .0005 vs control; #P < .05 and ##P < .005 vs low-PC. OD, optical density.
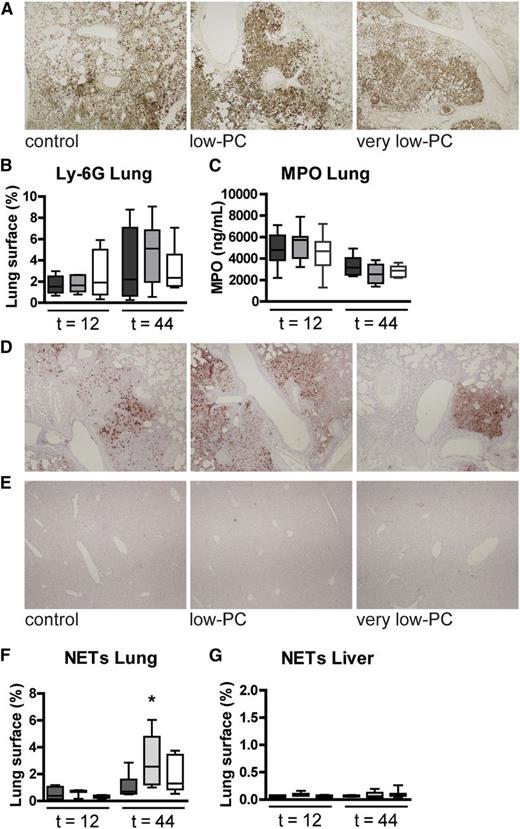
To determine the impact of thrombocytopenia on lung inflammation and pathology, we semiquantitatively scored lung histology slides obtained from naive and infected mice (Figure 3B,E). Despite increased bacterial loads in lungs of very low-PC and low-PC mice, no differences in histology scores or percentage infiltrated lung surface were found compared with control mice. Platelets have been reported to play a role in neutrophil recruitment to the lungs19,20 ; we therefore measured neutrophil influx in lungs by determining the number of Ly-6G-positive cells (Figure 4A-B) and by measuring myeloperoxidase (MPO) in whole lung homogenates (Figure 4C); differences between groups were not significant. Platelet attachment to neutrophils has been reported to be a threshold switch for release of neutrophil extracellular traps (NETs).21 Although NETs were not visible in lungs of uninfected mice, Klebsiella pneumonia resulted in enhanced NET formation. Low-PC mice showed increased NETs in their lungs at 44 hours after infection relative to infected control mice (P < .05; Figure 4D,F). NETs were not detected in the liver (Figure 4E,G).
Platelets are not required for lung neutrophil accumulation or NET formation in the lungs. Neutrophil accumulation in lung tissue was measured by quantification of Ly-6G stainings (A-B) and MPO measured in lung homogenates (C). NETs were stained using antibody to H3Cit for lung (D) and liver (E) and then quantified (F-G). Original magnification ×4 for panels A, D, and E; H&E stain. Ly-6G and NET positivity and total lung surface area were measured using Image J (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij), with the amount of Ly-6G or NET positivity expressed as a percentage of the total surface area. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation. n = 8 mice per group. *P < .05.
Platelets are not required for lung neutrophil accumulation or NET formation in the lungs. Neutrophil accumulation in lung tissue was measured by quantification of Ly-6G stainings (A-B) and MPO measured in lung homogenates (C). NETs were stained using antibody to H3Cit for lung (D) and liver (E) and then quantified (F-G). Original magnification ×4 for panels A, D, and E; H&E stain. Ly-6G and NET positivity and total lung surface area were measured using Image J (National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij), with the amount of Ly-6G or NET positivity expressed as a percentage of the total surface area. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation. n = 8 mice per group. *P < .05.
Platelets are not required for coagulation or endothelial cell activation during Klebsiella pneumosepsis
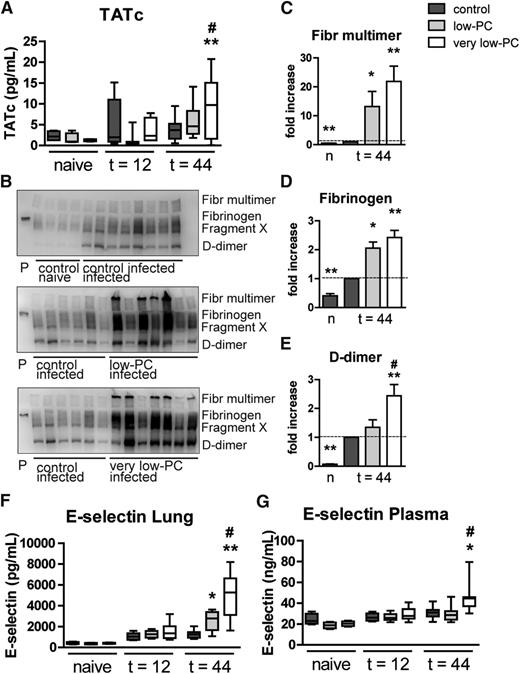
To obtain insight into the role of platelets in systemic coagulation activation during Klebsiella pneumonia–derived sepsis, we measured thrombin-antithrombin complex (TATc) levels in plasma of very low-PC, low-PC, and control mice. At 12 hours post infection, plasma TATc levels were not different between groups; remarkably, 44 hours after induction of pneumonia, very low-PC mice demonstrated increased plasma TATc concentrations compared with control (P < .005) and low-PC mice (P < .05; Figure 5A). To measure local coagulation in the lungs, we performed fibrin(ogen) western blotting on whole lung homogenates and quantified fibrinogen multimers and degradation products (fragment X and d-dimer; Figure 5B-E). Klebsiella pneumonia resulted in enhanced pulmonary coagulation in control mice relative to uninfected mice. Thrombocytopenia was associated with increased pulmonary deposition of fibrinogen multimers and split products in mice infected with Klebsiella via the airways, an effect that was PC dependent. We measured (soluble) E-selectin in plasma and in whole lung homogenates as parameters for systemic and local endothelial cell activation, respectively.22,23 In line with their exaggerated procoagulant response, very low-PC mice in particular showed strongly increased plasma and lung E-selectin levels (Figure 5F-G).
Platelets are not required for coagulation or endothelial cell activation during Klebsiella pneumosepsis. Very low-PC, low-PC, and control mice were infected with K pneumoniae via the airways and euthanized at 12 or 44 hours. (A) Plasma TATc levels were measured as a marker for systemic coagulation activation. (B) Fibrin(ogen) was detected by western blotting on lung homogenate samples. (C-E) Semiquantification of fibrin(ogen) blots; quantification scores of naive control mice, very low-PC, and low-PC mice were normalized to infected controls. E-selectin was measured in lung homogenates (F) and in plasma (G) as a marker for endothelial cell activation. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation, or as bars depicting mean ± standard error of the mean (SEM). n = 8 mice per group. *P < .05 and **P < .005, vs control; #P < .05 vs low-PC. Fibr, fibrinogen.
Platelets are not required for coagulation or endothelial cell activation during Klebsiella pneumosepsis. Very low-PC, low-PC, and control mice were infected with K pneumoniae via the airways and euthanized at 12 or 44 hours. (A) Plasma TATc levels were measured as a marker for systemic coagulation activation. (B) Fibrin(ogen) was detected by western blotting on lung homogenate samples. (C-E) Semiquantification of fibrin(ogen) blots; quantification scores of naive control mice, very low-PC, and low-PC mice were normalized to infected controls. E-selectin was measured in lung homogenates (F) and in plasma (G) as a marker for endothelial cell activation. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation, or as bars depicting mean ± standard error of the mean (SEM). n = 8 mice per group. *P < .05 and **P < .005, vs control; #P < .05 vs low-PC. Fibr, fibrinogen.
Thrombocytopenia has a bimodal PC-dependent effect on the occurrence of distant organ injury during Klebsiella-induced pneumosepsis
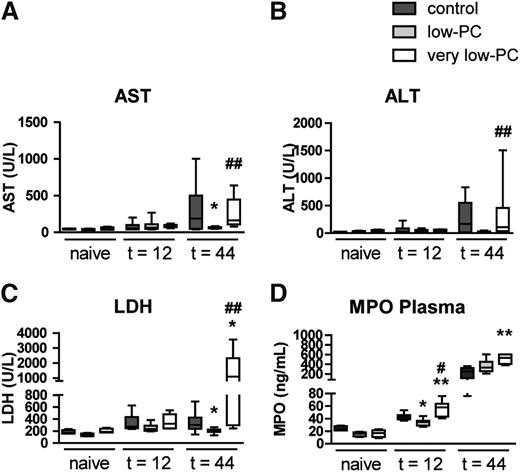
This model of pneumosepsis is associated with distant organ damage during late-stage infection.16,17 We measured plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) as measures of hepatocellular injury, and lactate dehydrogenase (LDH) as a marker of cellular injury in general (Figure 6A-C). As expected,16,17 plasma AST, ALT, and LDH levels increased significantly in control mice during the course of the infection. Interestingly, distant organ injury was reduced in low-PC mice (significant for AST and LDH); however, very low-PC mice were not different from control animals. Recently, NET formation in the liver was shown to contribute to liver damage after intraperitoneal infection with high-dose Eschericia coli21 ; however, we were unable to visualize NETs in liver tissue of infected mice in any of the experimental groups (Figure 4E,G). We speculated that platelets might influence systemic neutrophil activation24 and, therefore, measured plasma MPO levels (Figure 6D). While very low-PC mice had elevated plasma MPO levels relative to infected control mice, low-PC mice displayed an attenuated increase in plasma MPO at 12 hours post infection.
Thrombocytopenia has a bimodal PC-dependent effect on the occurrence of distal organ injury during Klebsiella-induced pneumosepsis. Very low-PC, low-PC, and control mice were infected with K pneumoniae via the airways and euthanized at 12 or 44 hours. AST (A) and ALT (B) were measured in plasma as markers for hepatocellular injury. (C) LDH was measured in plasma as a more general cell injury marker. (D) Systemic neutrophil activation was assessed by measuring MPO in plasma. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation, or as bars depicting mean ± SEM. n = 8 mice per group. *P < .05 and **P < .005 vs control; #P < .05 and ##P < .005 vs low-PC.
Thrombocytopenia has a bimodal PC-dependent effect on the occurrence of distal organ injury during Klebsiella-induced pneumosepsis. Very low-PC, low-PC, and control mice were infected with K pneumoniae via the airways and euthanized at 12 or 44 hours. AST (A) and ALT (B) were measured in plasma as markers for hepatocellular injury. (C) LDH was measured in plasma as a more general cell injury marker. (D) Systemic neutrophil activation was assessed by measuring MPO in plasma. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation, or as bars depicting mean ± SEM. n = 8 mice per group. *P < .05 and **P < .005 vs control; #P < .05 and ##P < .005 vs low-PC.
Thrombocytopenia enhances proinflammatory cytokine release during Klebsiella sepsis
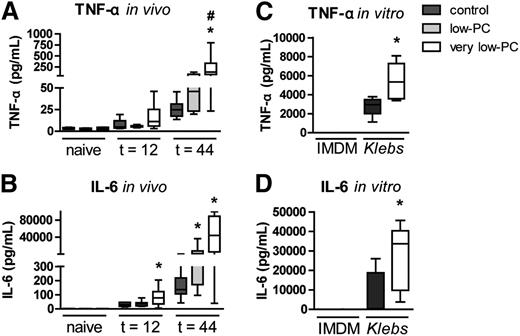
After infection with Klebsiella, thrombocytopenia was associated with elevated plasma tumor necrosis factor (TNF)-α and interleukin (IL)-6 relative to control mice, an effect that was clear at 44 hours and most pronounced in very low-PC mice (Figure 7A-B). Considering that this effect could be the consequence of the much higher bacterial loads in thrombocytopenic mice, we next incubated whole blood from uninfected very low-PC and control mice with viable Klebsiella and measured proinflammatory cytokine levels in supernatants after 6 hours. Klebsiella CFUs increased almost 105-fold during the 6-hour incubation period in blood of both very low-PC and control mice, illustrating the high virulence of this bacterium (supplemental Figure 2). Whole blood from very low-PC mice produced more TNF-α (P < .05) and IL-6 (P < .05) than whole blood from control mice (Figure 7C-D). Of note, thrombocytopenic mice also displayed higher cytokine levels in lungs after infection, especially during late-stage infection in very low-PC mice (supplemental Figure 3).
Thrombocytopenia enhances proinflammatory cytokine release during Klebsiella sepsis. Very low-PC, low-PC, and control mice were infected with K pneumoniae via the airways and euthanized at 12 or 44 hours. TNF-α (A) and IL-6 (B) were measured in plasma. Heparinized whole blood obtained from naive very low-PC and control mice was diluted in IMDM (1:1) and incubated with Klebsiella or IMDM control for 6 hours. TNF-α (C) and IL-6 (D) were measured in plasma supernatant. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation, n = 8 (infected) and n = 6 (naive) mice per group. *P < .05 vs control; #P < .05 vs low-PC.
Thrombocytopenia enhances proinflammatory cytokine release during Klebsiella sepsis. Very low-PC, low-PC, and control mice were infected with K pneumoniae via the airways and euthanized at 12 or 44 hours. TNF-α (A) and IL-6 (B) were measured in plasma. Heparinized whole blood obtained from naive very low-PC and control mice was diluted in IMDM (1:1) and incubated with Klebsiella or IMDM control for 6 hours. TNF-α (C) and IL-6 (D) were measured in plasma supernatant. Data are expressed as box-and-whisker plots depicting the smallest observation, lower quartile, median, upper quartile, and largest observation, n = 8 (infected) and n = 6 (naive) mice per group. *P < .05 vs control; #P < .05 vs low-PC.
Discussion
We investigated the role of platelets during gram-negative pneumonia–induced sepsis by infecting thrombocytopenic and control mice with the common human sepsis pathogen K pneumoniae via the airways. Our main findings were that thrombocytopenia enhanced mortality during Klebsiella-induced pneumosepsis, and that even in mice with severe platelet depletion, a modest rise in PC resulted in prolonged survival. In accordance, we found a PC-dependent enhanced bacterial growth in the lungs, blood, and distant organs in thrombocytopenic mice during late-stage sepsis. Very low PCs (<5 × 109/L) resulted in hemorrhage at the primary site of infection but not in distant organs in septic mice; PCs of 5 to 13 × 109/L during the course of the infection were sufficient to largely maintain hemostasis. Low PCs additionally resulted in attenuated distant organ injury during gram-negative sepsis. In both our in vivo and in vitro models, thrombocytopenia increased the release of proinflammatory cytokines to Klebsiella.
We induced thrombocytopenia of two distinct severities to investigate the hemostatic and immunomodulatory role of platelets during infection. PCs of 5 to 13 × 109/L would be sufficient to maintain hemostasis under inflammatory conditions10 ; however, PCs in that range would hypothetically be insufficient to perform platelets’ immune functions. Notably, the thrombocytopenia that occurs in critically ill patients is usually less severe than induced here and in previous mouse studies examining the role of platelets in hemostasis and inflammation.10,25-27 Hence, our results should be viewed in the context of an established and controlled mouse sepsis model, whereas sepsis patients represent a much more heterogeneous group with many more variables, as illustrated by differential genomic responses in blood leukocytes in experimental mouse sepsis and clinical human sepsis.28
We used a clinically relevant model of sepsis induced by a low inoculum (104 CFUs) of a virulent pathogen, which was associated with a gradually growing bacterial load at the primary site of infection and which resulted in dissemination of the infection to distant organs during later stages as well as systemic inflammation and injury. This model, which is associated with clear sickness behavior such as detailed in the clinical scoring method, allowed us to study the role of platelets in distinct phases of the host response to infection; that is, in an early phase during which innate immunity serves a protective role, and in a later stage during which abundant inflammation may result in tissue injury. We considered lungs to be the most appropriate primary source of infection because pneumonia is the most common cause of sepsis2,3 and because platelets in particular exert proinflammatory and immunomodulatory effects in the lungs.29 In addition, this model permitted careful analyses of the role of platelets in vascular integrity at various body sites during sepsis. Previous investigations that addressed the role of platelets in the host response to sepsis used very high infectious doses of Escherichia coli (107 CFUs) administered via the intraperitoneal route, resulting in an immediate fulminant septic shock syndrome resembling endotoxic shock.13,21 One of these studies examined the role of platelets in antibacterial defense, reporting elevated bacterial loads in blood of thrombocytopenic mice but not at the primary site of infection (the peritoneal cavity).21 Similarly, thrombocytopenic mice had higher bacterial loads in their blood 1 and 4 hours after intravenous injection of high-dose Bacillus cereus (5 × 107 CFUs), a bacterial challenge that was rapidly cleared by mice with normal PCs.30 The Klebsiella strain used here is much more virulent, as illustrated by the high mortality rates after infection with a relatively low bacterial dose (Figure 2) and the logarithmic growth of this pathogen in whole blood (supplemental Figure 2). We showed that the presence of platelets in blood did not inhibit bacterial growth in vitro. Notably, in vivo, PCs of only 5 to 13 × 109/L conferred significant improvement of antibacterial defense, as illustrated by the different bacterial loads between low-PC and very low-PC mice. Although aspirin treatment increased mortality and inflammation in high-dose lipopolysaccharide (LPS)- or E coli–induced inflammation,13 secondary platelet activation played no role in our Klebsiella pneumosepsis model. Treatment with clopidogrel, an irreversible inhibitor of adenosine 5′-diphosphate platelet activation,31 reduced rather than enhanced bacterial loads in distant organs in this model of gram-negative sepsis (S.F.d.S., C.v.t.V., T.A.M.C., B.J.A.A., J.J.T.H.R., and T.v.d.P., unpublished data). Others have found that clopidogrel pretreatment attenuated the drop in PC and improved end-organ damage in polymicrobial sepsis in mice.32 In contrast, clopidogrel treatment did impact outcome parameters in E coli endotoxin–infused pigs.33 The discrepancies between the aforementioned studies are likely due to differences between the models or other anti-inflammatory effects of aspirin or clopidogrel.
Platelets play a crucial role in protection of the vascular integrity during inflammation.10,18,34 Although thrombocytopenia per se does not induce bleeding, locally instilled inflammatory stimuli caused hemorrhages in skin, brain, and lungs in thrombocytopenic mice.10 The present study is the first to investigate the role of platelets in hemostasis in different organs during sepsis. Our results indicate that platelets are important in maintenance of hemostasis at the primary site of infection only, despite bacterial dissemination and evidence for distant inflammatory organ injury. Indeed, even in mice with very low-PC (<5 × 109/L of normal), liver and spleen did not show any sign of hemorrhage. Possibly, the high bacterial loads in distant organs would have resulted in inflammation-induced hemorrhage in thrombocytopenic mice after longer infection durations; earlier deaths precluded such analyses. Hemostasis in the lungs was largely preserved in low-PC mice, with median PCs of 5 × 109/L and 13 × 109/L (which is 1 to 2% and 9% of normal PC) at 12 and 44 hours after infection, respectively. In a previous study, transfusion of platelets into thrombocytopenic mice to PCs of 4% to 8% of normal significantly reduced skin bleeding in a model of the reverse passive Arthus reaction, whereas platelet transfusion to PCs of 10% to 15% of normal were required to completely prevent bleeding.10 Together, these data suggest that the absolute threshold at which thrombocytopenia results in bleeding may differ depending on body site and type of inflammatory challenge. In this respect, it is important to note that previous studies examining the impact of thrombocytopenia on inflammation-induced hemorrhage used noninfectious stimuli to cause acute inflammation,10,34 whereas our study examined thrombocytopenia in the setting of a gradually evolving inflammatory response elicited by a progressively expanding bacterial load.
Platelets provide a phospholipid surface for catalysis of tissue factor (factor VIIa)-mediated coagulation.35 The coagulation cascade was, however, not hindered by PCs or very low PCs in our model of pneumonia-derived sepsis. On the contrary, very low-PC mice in particular displayed enhanced coagulation activation, both in plasma and in lungs. Moreover, platelets can contribute to endothelial cell activation and vascular inflammation,36 which are hallmarks of sepsis.37 Using E-selectin as a marker for endothelial cell activation,22,23 we showed that platelets are not essential for this response during gram-negative sepsis. Very low-PC mice even demonstrated evidence of increased endothelial cell activation. Together, these results suggest that coagulation and endothelial cell activation are driven by the systemic inflammatory response during sepsis, with platelets playing a limited role. It is likely that thrombocytopenia-induced hemorrhage promotes coagulation activation, resulting in thrombin generation, which secondarily contributes to endothelial cell activation via protease activated receptors 1 and 2. Additionally, the exaggerated systemic inflammation in severe thrombocytopenic mice provides an explanation for the enhanced procoagulant and endothelial cell responses in these animals.
Platelet activation is known to augment inflammatory responses,11,12 and platelet depletion has been described to be protective in sterile LPS-induced lung inflammation.25,26 Despite ≥100-fold higher bacterial burdens and macroscopic bleeding in the lungs of very low-PC mice 44 hours after Klebsiella infection, the extent of lung damage and inflammation was similar between groups, and pulmonary neutrophil numbers were similar in thrombocytopenic and control mice. Previous studies on acute lung inflammation did reveal a role for platelets in neutrophil recruitment.29,38 Our data suggest that the influence of platelets on neutrophil influx to sites of lung inflammation likely depends on the stimulus and the time during which this inflammatory stimulus emerges in the airways. Although platelets have been implicated in NET formation,21,39 we did not find firm evidence for such a role in the lungs of mice infected with Klebsiella. One might argue, however, that in very low-PC mice, NET formation was relatively impaired at 44 hours post infection compared with low-PC and control mice, considering the very high bacterial burdens in very low-PC mice.
Previous studies showed increased organ failure during endotoxin shock or after B cereus infusion in thrombocytopenic mice.13,30 In accordance, very low-PC mice in the present study displayed increased LDH plasma levels during Klebsiella pneumosepsis. Interestingly, low-PC mice were protected from distant organ injury and showed significant reduction in transaminases and LDH released in plasma. In fulminant acute E coli sepsis, platelets were shown to contribute to liver injury at least in part through enhancing NET formation21 ; however, in our more gradually evolving sepsis model, we did not detect NETs in liver tissue. Interestingly, low-PC but not very low-PC mice had attenuated systemic neutrophil activation 12 hours after infection, suggesting that platelets may inhibit neutrophil degranulation, an effect that is overruled by the high bacterial burdens in very low-PC mice. Possibly, the reduced systemic neutrophil degranulation in low PC-mice might explain the diminished hepatocellular injury in this group. Other platelet-mediated mechanisms for organ injury during sepsis could be via microthrombosis and ischemia,21,40 induction of apoptosis,41 or Granzyme-B–mediated toxicity,42 or by shedding of platelet microparticles.43-45 The protective role of platelets may not be restricted to gram-negative pneumonia-derived sepsis. Our own preliminary data suggest that severe thrombocytopenia results in enhanced bacterial dissemination during pneumonia caused by Streptococcus pneumoniae (data not shown); moreover, in lymphocytic choriomeningitis virus infection, profound platelet depletion caused systemic bleeding and death, whereas nonhemorrhagic and partially platelet-depleted mice were unable to control viral replication.27
The regulatory role of platelets in cytokine response is still elusive. Systemic TNF-α release in response to low-dose intravenous LPS was impaired in thrombocytopenic mice and restored after platelet transfusion.46 To the contrary, platelets in complex with macrophages inhibited TNF-α and IL-1β production after high-dose intravenous LPS or E coli challenges.13 Additionally, platelet GpIb-IX had suppressive effects on inflammation in a cecal ligation and puncture model.47 Although platelets potentiated macrophages to produce TNF-α and IL-6 after low-dose (100 pg/mL) LPS stimulation in vitro,48 high-dose LPS stimulation (50-10 000 ng/mL) induced platelet-macrophage interaction that inhibited TNF-α production.13,14 In our studies, in which high levels of LPS-expressing K pneumoniae were present, cytokine production was enhanced in vivo and after whole blood stimulation in vitro. Although initial cytokine production is indispensable for an adequate host response to invading pathogens, excessive cytokine production might have the opposite effect during severe infection.49 Platelets might serve as a switch for the inflammatory response during sepsis; that is, by promoting inflammation at an early stage of infection when the inflammatory stimulus is low and by inhibiting cytokine production when inflammatory stimuli become high.13
In conclusion, severe thrombocytopenia is associated with a strongly impaired host defense during pneumonia-derived gram-negative sepsis, which is proportional to the extent of platelet depletion. While in sepsis, patients’ low PCs could merely be a marker of disease severity; however, our current results suggest that thrombocytopenia plays a causative role in increased sepsis mortality.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Marieke ten Brink, Joost Daalhuisen, Michael Tanck, and Chi Hau for their technical assistance. This work was supported by an Academic Medical Center Graduate School Scholarship (S.F.d.S.) and a grant from the Landsteiner Foundation for Blood Transfusion Research (LSBR 1309) (T.A.M.C.).
Authorship
Contribution: S.F.d.S., T.A.M.C., and B.J.A.A. performed experiments; J.J.T.H.R. analyzed results; S.F.d.S., C.v.t.V., and T.v.d.P. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sacha F. de Stoppelaar, Center for Experimental and Molecular Medicine, Academic Medical Center, Meibergdreef 9, Room G2-130, 1105 AZ Amsterdam, The Netherlands; e-mail: s.f.destoppelaar@amc.uva.nl.