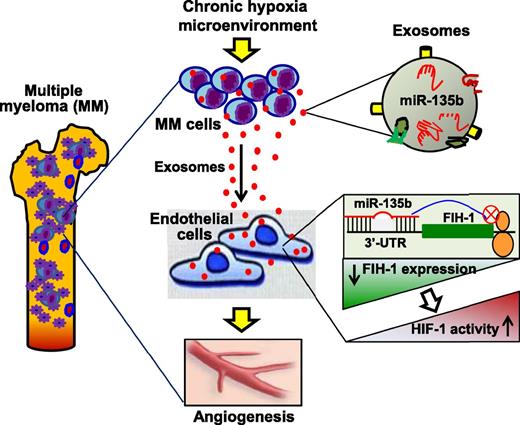
In this issue of Blood, Umezu et al identified that exosomal miR-135b plays a critical role in mediating multiple myeloma (MM) cell-to-endothelial cell communication under chronic hypoxia.1
Under a long-term hypoxia microenvironment, MM cells actively secrete miR-135b–enriched exosomes, which are taken up by the surrounding endothelial cells, leading to reduced expression of FIH-1, one of the miR-135b targets that inhibits the activation of HIF-1. Consequently, the HIF-1 activity is increased in recipient ECs, resulting in an accelerated angiogenesis that may promote MM-cell survival, proliferation, and migration.
Under a long-term hypoxia microenvironment, MM cells actively secrete miR-135b–enriched exosomes, which are taken up by the surrounding endothelial cells, leading to reduced expression of FIH-1, one of the miR-135b targets that inhibits the activation of HIF-1. Consequently, the HIF-1 activity is increased in recipient ECs, resulting in an accelerated angiogenesis that may promote MM-cell survival, proliferation, and migration.
Multiple myeloma is 1 type of the most common blood cancers characterized by clonal proliferation of malignant white blood cells that are home to the bone marrow. It accounts for approximately 13% of hematologic disorders and causes >63 000 deaths per year worldwide.2 Although the current treatment (ie, autologous hematopoietic stem-cell transplantation) may prolong the patient’s survival, MM remains incurable for the majority of MM patients because of relapse.3 Thus, innovations in MM treatment are desperately needed. Over the past 2 decades, major progress in the field of MM research has been made in understanding the importance of crosstalk between MM cells and the surrounding microenvironment.4 It is now well appreciated that hypoxia occurs in MM-infiltrated bone marrow, which promotes MM-cell proliferation and migration and the angiogenic activity of endothelial cells. However, the underlying mechanism remains obscure. In this work, Umezu et al identified a novel mode of exosomal miR-135b–mediated interaction between MM cells and surrounding endothelial cells.1
Exosomes are a group of nanometer-sized membrane vesicles (30-100 nm) released from numerous cell types.5 Although they are generated constitutively in cells, stress or disease conditions can stimulate exosome biogenesis and release.6-8 Furthermore, it is well recognized that protein and RNA sorting into exosomes is highly regulated by various pathophysiologic stress stimuli, which are reflective of their parent-cell status.8 Through shuttling of a specific set of exosome contents into target cells, the surrounding microenvironment of parent cells is modulated to be either friend or foe. The authors in this work tried to determine how MM cells induce adaptive mechanisms that serve to promote the survival and dissemination of malignant cells. One of the important aspects is that the authors incubated MM cells under 1% O2 for 6 to 7 months and established hypoxia-resistant (HR) cell lines to mimic in vivo chronic hypoxic conditions that occur in MM patients. Using these HR-MM cell lines, they were able to collect and characterize authentic hypoxic exosomes. The findings of Umezu et al indicate that HR-MM cells have a capacity to secrete more exosomes and, importantly, such exosomes can promote human umbilical vein endothelial cell (HUVEC) tube formation under both normoxic conditions (20% O2, remote effect) and hypoxic conditions (1% O2, local effect). Thus, hypoxic MM cells may modulate their microenvironment to enhance angiogenic potential by secretion of exosomes (see figure).
Recently, miRNAs have been implicated as critical factors in exosomes because they largely decide exosome functional consequences in recipient cells.7,9,10 To dissect how hypoxic exosomes released from MM cells promote angiogenesis, Umezu et al profiled miRNA expression in 3 lines of HR-MM cells and their released exosomes. They observed that the levels of miR-210 and miR-135b were consistently upregulated in both acute and chronic hypoxia-treated MM, and also were highly encased in exosomes released from these cells. However, the authors further identified that high levels of miR-210 were only maintained in hypoxic culture and gradually disappeared in the normoxic condition. By contrast, upregulation of miR-135b could be maintained in the normoxic condition. On the basis of these findings, Umezu et al suggest that miR-210 is a universal hypoxia-responsive miRNA with transient effect, whereas miR-135b is an HR-MM-cell–specific miRNA with chronic effect. Given that exosomes are able to shuttle their contents between cells,10 the authors also provided convincing evidence showing that exosomal miR-135b could be effectively delivered to HUVECs and functionally targeted to the 3′-UTR of the factor-inhibiting hypoxia-inducible factor-1 (HIF-1) (FIH-1) gene, leading to reduced expression of FIH-1 (see figure). FIH-1 is an asparaginyl hydroxylase enzyme that inhibits the transcriptional activity of HIF-1. Accordingly, the HIF-1 activity was dramatically increased in hypoxic exosome–treated HUVECs. Therefore, this work by Umezu et al suggests that hypoxia-driven, accelerated angiogenesis is ascribed to exosomal miR-135b shed from HR-MM cells by targeting the FIH-1/HIF-1 signaling pathway (see figure).
It should be commended that Umezu et al provide compelling in vivo and in vitro evidence showing that knockdown of miR-135b in HR-MM exosomes dampened their proangiogenic effects. However, a note of caution should be added: exosomes derived from chronic hypoxia MM cells might encapsulate many specific proteins (ie, receptors and kinases), mRNAs, and miRNAs. In fact, the authors have presented in this study that chronic hypoxia MM-exosomes encase tens of other miRNAs in addition to miR-135b. It is therefore possible, if not likely, that other exosomal contents may additionally contribute to the enhanced angiogenesis in MM.
Finally, the authors nicely made an effort to characterize exosomes from the primary myeloma cells of 2 MM patients. Although their findings indicate that miR-135b levels were elevated in both exosomes and parental myeloma cells from one of the MM patients, the authors could not define any association between upregulation of exosomal miR-135b and therapy-resistant MM patients with this limited number of MM patients. Another puzzling issue is that, even when MM cells exhibit high levels of miR-135b, its levels in plasma are very low. The plausible interpretation Umezu et al provided in this study is that exosomal miR-135b might play a role in local niche rather than circulation. Thus, future studies will need to clarify the clinical significance of exosomal miR-135b in a large number of MM patients. Clinically, it will be very interesting to explore in the future whether blockade of exosome biogenesis/release in MM cells can improve patients’ survival.
Overall, this work by Umezu et al is exciting because they report for the first time that under chronic hypoxia conditions, MM cells enhance angiogenesis through the exosomal transfer of miR-135b to endothelial cells, resulting in a reduced expression of FIH-1 and increased activity of HIF-1 (see figure).
Conflict-of-interest disclosure: The author declares no competing financial interests.