Abstract
Pyruvate kinase deficiency (PKD) is an autosomal recessive enzymopathy that is the most common cause of hereditary nonspherocytic hemolytic anemia (HNSHA). PKD is a rare disease characterized by a life-long chronic hemolysis with severe co-morbidities. It is hypothesized that insufficient energy production to maintain red cell membrane homeostasis promotes the chronic hemolysis. Treatment is generally palliative, focusing on the resultant anemia, and there are no approved drugs that directly target mutated pyruvate kinase.
AG-348 is an allosteric activator of the red cell isoform of pyruvate kinase (PKR) that has recently entered Phase I clinical trials in normal healthy volunteers. AG-348 increases the catalytic efficiency and enhances the protein stability of a spectrum of recombinantly expressed PKR mutant proteins that have been associated with PKD. PKD red cells are characterized by changes in metabolism associated with defective glycolysis, including a build-up of the upstream glycolytic intermediate 2,3-DPG and deficiency in the PKR product adenosine triphosphate (ATP). PKR flux, e.g. the rate of carbon flow through the PKR enzyme reaction, was examined in PKD patient or wild type (WT) donor blood samples by incubation of whole blood with a stable isotope tracer, [U-13C6]-glucose. At various time points after the addition of [U-13C6]-glucose, metabolism was quenched and metabolites were extracted. Metabolite pool sizes and 13C label incorporation into glycolytic intermediates were monitored by LC/MS. The rate of label incorporation was found to be significantly slower in PKD patient red cells, consistent with decreased glycolytic activity. Treatment of PKD red cells with AG-348 ex-vivo induces changes in metabolism consistent with increased glycolytic activity including reduced 2,3-DPG levels, increased ATP levels, and increased PKR enzyme activity levels.
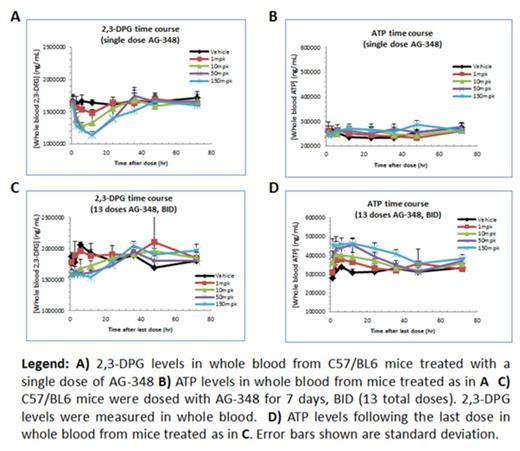
The effect of AG-348 on red cell metabolism in vivo was evaluated in mice. C57/BL6 mice were dosed by oral gavage either with a single dose, or with multiple doses (BID) of AG-348 for 7 days. Dose levels tested were 1 mpk, 10 mpk, 50 mpk, and 150 mpk. Following the last dose, mice were bled to evaluate drug exposure and pharmacodynamic markers including 2,3-DPG and ATP levels, and PKR activity. AG-348 was demonstrated to be a well-behaved compound, with dose-proportional increase in exposure, both in the single-dose and multiple dose studies. A single dose of AG-348 resulted in a dose-dependent increase in PKR activity levels, concomitant with reduction in 2,3-DPG levels. There were no significant changes in ATP levels after a single administration of AG-348. In the multiple-dose studies, similar changes in PKR activity and 2,3-DPG levels were observed. In contrast to the single-dose study, ATP levels were observed to be robustly increased in a dose-dependent manner.
The effect of AG-348 on PKR flux was assessed in whole blood from mice treated with AG-348. C57BL/6 mice were dosed by oral gavage with AG-348 (150 mg/kg twice daily [BID]) for 3 days. Whole blood was incubated with [U-13C6]-glucose and the metabolite pool sizes and rate of 13C label incorporation into glycolytic intermediates were assessed. The data were subsequently analyzed using a mathematical model to quantify flux through the PKR reaction and it was determined that AG-348 treatment significantly increased flux through the PKR reaction.
Collectively, these data demonstrate that AG-348 not only potently binds to and activates the PKR enzyme in vivo, but this enzyme activation induces enhanced glycolytic pathway activity in red cells that results in profound changes in cellular metabolism, as reflected in dramatically increased ATP levels and reduced 2,3-DPG levels. As AG-348 has similar potency against the WT PKR enzyme as against tested mutant PKR enzymes in vitro, these data support the hypothesis that AG-348 treatment may similarly enhance glycolytic activity in PKD patients and thus correct the underlying pathology of PKD.
Kung:Agios Pharmaceuticals: Employment, Stockholder Other. Hill:Agios Pharmaceuticals: Employment, Stockholder Other. Chen:Agios Pharmaceuticals: Employment, Stockholder Other. Jha:Agios Pharmaceuticals: Employment, Stockholder Other. Kosinski:Agios Pharmaceuticals: Employment, Stockholder Other. Clasquin:Agios Pharmaceuticals: Employment, Stockholder Other. Si:Agios Pharmaceuticals: Employment, Stockholder Other. Kim:Agios Pharmaceuticals: Employment, Stockholder Other. Hixon:Agios Pharmaceuticals: Employment, Stockholder Other. Dang:A: Employment, Stockholder Other. Agresta:Agios Pharmaceuticals: Employment, Stockholder Other. Silverman:Agios Pharmaceuticals: Employment, Stockholder Other. Yang:Agios Pharmaceuticals: Employment, Stockholder Other.
Author notes
Asterisk with author names denotes non-ASH members.