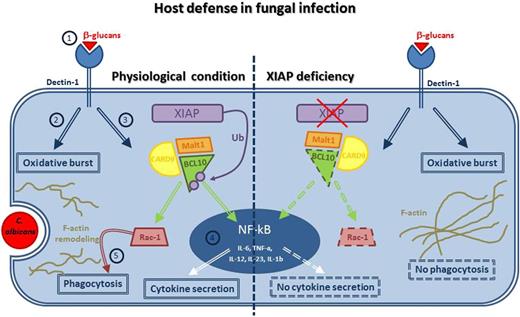
In this issue of Blood, Hsieh et al provided the molecular mechanism showing how X-linked inhibitor of apoptosis protein (XIAP) deficiency may lead to selective innate immunodeficiency recalling X-linked lymphoproliferative syndrome type 2 (XLP-2).1
In physiological conditions, engagement of dectin-1 by β-glucans composing the fungi wall (1) triggers a complex signaling pathway mediating the oxidative burst (2) and the CARD9/BCL10/Malt1 complex formation (3), which in turn activates NF-κB, with secretion of proinflammatory cytokines (4), and Rac-1, inducing F-actin remodeling and mediating phagocytosis (5). Collectively, these processes lead to the pathogen clearance and consequently to the switching off of the inflammatory burst. In XIAP deficiency, dectin-1–induced innate responses is impaired. BCL10 fails to activate on one side, NF-κB, resulting in no secretion of NF-κB–mediated proinflammatory cytokines, and on the other side, Rac-1, resulting in no phagocytosis. Persistence of C albicans results in overactivation of macrophages, excess production of inflammatory cytokines, and hemophagocytic syndrome. Dashed lines represent the inactive molecules/processes of the pathway.
In physiological conditions, engagement of dectin-1 by β-glucans composing the fungi wall (1) triggers a complex signaling pathway mediating the oxidative burst (2) and the CARD9/BCL10/Malt1 complex formation (3), which in turn activates NF-κB, with secretion of proinflammatory cytokines (4), and Rac-1, inducing F-actin remodeling and mediating phagocytosis (5). Collectively, these processes lead to the pathogen clearance and consequently to the switching off of the inflammatory burst. In XIAP deficiency, dectin-1–induced innate responses is impaired. BCL10 fails to activate on one side, NF-κB, resulting in no secretion of NF-κB–mediated proinflammatory cytokines, and on the other side, Rac-1, resulting in no phagocytosis. Persistence of C albicans results in overactivation of macrophages, excess production of inflammatory cytokines, and hemophagocytic syndrome. Dashed lines represent the inactive molecules/processes of the pathway.
XLP-2 is a lymphoproliferative disease associated with XIAP deficiency.2 Clinical symptoms are mostly attributed to the aberrant activation of macrophages and dendritic cells and the accumulation of activated T lymphocytes, often in response to Epstein-Barr virus infection.3 This hyperactivation of immune cells leads to hemophagocytic lymphohistiocytosis (HLH), an inflammatory disorder caused by the excess of cytokine production from hyperactivated lymphocytes and macrophages. XLP-2 is also associated with recurrent splenomegaly and fever, but mechanisms underlying the disease are largely misunderstood. Intriguingly, XIAP deficiency may cooperate with other genetic defects of innate immunity to induce the HLH picture.4
In this study, the authors show that XIAP-deficient mice are unable to resolve Candida albicans infections and confirm this defect in XIAP-deficient human macrophages. Moreover, they show that XIAP is required for the innate responses induced by dectin-1, a receptor belonging to the C-type lectin family and mainly expressed in dendritic cells and macrophages. Dectin-1 recognizes β-glucans that are key cell wall components of several fungi including C albicans. On ligand binding, dectin-1 transduces signals through its immunoreceptor tyrosine-based activation motif in the cytoplasmic domain and activates the caspase recruitment domain family member 9 (CARD9)–BCL10–nuclear factor (NF-κB) axis, resulting in the activation of several genes including those encoding proinflammatory cytokines (see figure). Because of the defective activation of this pathway, XIAP−/− mice are unable to clear the infection by C albicans, whose persistence results in overactivation of macrophages, excessive production of inflammatory cytokines, and splenomegaly. Mice die after 15 days from candida administration. A similar picture is displayed by mice deficient of dectin-1 or CARD-9.
Beside this, Hsieh et al describe a number of important novel observations. They show that XIAP binds and polyubiquitinates BCL10, which is required for the dectin-1–induced innate response and activation of NF-κB. BCL10 contributes to adaptive and innate immunity through the assembly of a signaling complex that plays a key role in the activation of NF-κB triggered through the antigen receptor or FcRs. In XIAP-deficient mice, BCL10 polyubiquitination is missing, and this in turn impaired dectin-1–induced BCL10-mediated activation signals. Interestingly, treatment of XIAP mice with curdlan, a dectin-1 ligand, induced an XLP-2 like syndrome, recalling the effects of C albicans infection.
Moreover, a recent study showed that BCL10 has an NF-κB–independent role in human macrophages, and depletion of BCL10 impairs Rac1 activation, F-actin remodeling, and phagosome formation.5 Hsieh et al found that dectin-1–induced Rac1 activation is attenuated in mouse and human XIAP-deficient macrophages. Interestingly, they demonstrated that impaired uptake of C albicans by XIAP-deficient macrophages is restored by expression of active Rac1. These results suggest that drugs improving phagocytosis in XIAP macrophages may exert therapeutic effects in XLP2. To this aim, Hsieh et al evaluate the effect of resolvin D1, an anti-inflammatory molecule enhancing macrophage phagocytosis, and show that in vivo administration, 3 days after C albicans infection, fully rescues XIAP-deficient mice from disease and death.6 Moreover, resolvin D1 restores Rac1 activation and phagocytosis in XIAP-deficient macrophages and results in effective resolution of inflammation and elimination of C albicans.
Two other interesting points elucidated by this study are as follows. First, these results explain why, when myeloablative conditioning regimens are used in allogeneic hematopoietic cell transplantation in XIAP-mutated patients, a poor survival has been recorded.7 In fact, XIAP deficiency already impairs the innate reactivity of myeloid cells, and their depletion further aggravates the innate immunodeficiency. Second, involvement of XIAP in BCL10-mediated NF-κB activation may also be relevant for other pathologic conditions such as autoinflammatory syndromes. It has been shown that a common missense polymorphism of the XIAP gene (423Q), associated with recurrent fever, increases XIAP expression, which may influence secretion of tumor necrosis factor α and interleukin-1β by macrophages.8 It is intriguing that defective expression of XIAP can cause selective innate immunodeficiency, whereas its increased expression may favor development of a hyperimmune disease. Therefore, targeting of BCL10 may have therapeutic benefits in different pathological conditions.
Conflict-of-interest disclosure: The author declares no competing financial interests.