Key Points
SK2 is overexpressed in myeloma cells and contributes to myeloma cell survival and proliferation.
SK2-specific inhibitor promotes proteasome degradation of Mcl-1 and c-Myc and inhibits myeloma growth in vitro and in vivo.
Abstract
Sphingolipid metabolism is being increasingly recognized as a key pathway in regulating cancer cell survival and proliferation. However, very little is known about its role in multiple myeloma (MM). We investigated the potential of targeting sphingosine kinase 2 (SK2) for the treatment of MM. We found that SK2 was overexpressed in MM cell lines and in primary human bone marrow (BM) CD138+ myeloma cells. Inhibition of SK2 by SK2-specific short hairpin RNA or ABC294640 (a SK2 specific inhibitor) effectively inhibited myeloma cell proliferation and induced caspase 3–mediated apoptosis. ABC294640 inhibited primary human CD138+ myeloma cells with the same efficacy as with MM cell lines. ABC294640 effectively induced apoptosis of myeloma cells, even in the presence of BM stromal cells. Furthermore, we found that ABC294640 downregulated the expression of pS6 and directed c-Myc and myeloid cell leukemia 1 (Mcl-1) for proteasome degradation. In addition, ABC294640 increased Noxa gene transcription and protein expression. ABC294640, per se, did not affect the expression of B-cell lymphoma 2 (Bcl-2), but acted synergistically with ABT-737 (a Bcl-2 inhibitor) in inducing myeloma cell death. ABC294640 suppressed myeloma tumor growth in vivo in mouse myeloma xenograft models. Our data demonstrated that SK2 provides a novel therapeutic target for the treatment of MM. This trial was registered at www.clinicaltrials.gov as #NCT01410981.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy in the United States, where it accounts for about 11 000 deaths annually.1,2 The overall outcome and survival of patients with MM have significantly improved over the last decade, largely due to the use of several highly active agents (ie, thalidomide, lenalidomide, and bortezomib) and the incorporation of high-dose chemotherapy supported with autologous hematopoietic stem cell transplantation. MM, however, remains an incurable disease. Patients may relapse within months after autologous hematopoietic stem cell transplantation. Furthermore, nearly all MM patients will eventually develop resistance to the agents currently available. There is an unmet medical need for the development of novel therapeutic agents for this disease. It is particularly important to develop new agents that do not share a similar mechanism of action with proteasome inhibitors or immunomodulatory drugs because most of the refractory/relapsed MM patients would have been exposed to those agents during their course of treatment.
Sphingolipids are an extremely diverse group of water insoluble molecules that include ceramides, sphingoid bases, ceramide phosphates and sphingoid-base phosphates. In addition to supporting the structure and fluidity of the lipid bilayer, sphingolipid metabolites function as second messengers and hormones, and regulate cytokine-mediated cell signaling.3,4 Sphingolipids are involved in a wide range of biological and pathological events including inflammation, cell proliferation, apoptosis, angiogenesis, and transformation (reviewed in Snider et al,5 Nixon,6 Maceyka et al,7 Cowart,8 Saddoughi et al,9 and Billich and Baumruker10 ). More recently, sphingolipid metabolism is being increasingly recognized as a key pathway in tumor cell survival and in cancer biology.11-18
Among sphingolipid metabolites, ceramide, sphingosine, and sphingosine-1-phosphate (S1P) are the key players for their biophysiological functions. Ceramide can be produced via hydrolyzation of sphingomyelin in response to stimuli such as cytokines and growth factors. Ceramide is further hydrolyzed to sphingosine. Then sphingosine is rapidly phosphorylated by sphingosine kinases (SKs) to S1P. Ceramide and sphingosine are proapoptotic, inducing apoptosis in tumor cells without disrupting quiescent normal cells.19-22 In contrast, S1P is mitogenic and antiapoptotic. A critical balance (ie, a ceramide:S1P rheostat) is hypothesized to determine the fate of the cell.12,23,24
There is accumulating evidence demonstrating an important role of S1P in cancer cell survival,25,26 drug resistance,27 adhesion,28,29 and the communication between tumor cells and the microenvironment.30 Most effort has been focused on developing modulators of S1P receptors, such as Fingolimod (FTY720). FTY720 was found to be able to induce apoptosis and overcome drug resistance in MM.25 In a fundamentally different approach, our current study targeted SKs that catalyze the generation of S1P. We reasoned that SKs provide a potential site for manipulation of the ceramide:S1P rheostat. SKs have 2 isoenzymes: sphingosine kinase 1 (SK1) and sphingosine kinase 2 (SK2). SK1 was found to play a key role in IL-6 induced myeloma cell proliferation and survival.25-27,31 Many studies have suggested that the biological roles and localization of SK1 and SK2 are different,5,17,32-35 and very little is known about the role of SK2 in MM. Herein, we examined the role of SK2 in myeloma cell survival and determined the potential of targeting SK2 for the treatment of MM.
Methods and materials
Cell lines
Patient samples and isolation of primary human CD138+ myeloma cells
Institutional Review Board approval, patient bone marrow (BM) aspirates, and isolation of CD138+ myeloma cells were described in the supplemental Methods.
Reagents
ABC294640 (the SK2-specific inhibitor) was synthesized and provided by Apogee Biotechnology Corp. SK1 inhibitor (SK1-II) (ie, 2-[p-hydroxyanilino]-4-[p-chlorophenyl]thiazole) was purchased from Echelos Biosciences, Inc. (Salt Lake City, UT). For other reagents, please see the supplemental Methods.
SK2-specific shRNA lentiviruses
Details of short hairpin RNA (shRNA) lentivirus production38 and transduction were available in the supplemental Methods.
In vitro drug treatment, cell proliferation assay, apoptosis assay, western blot analysis, and reverse transcription-polymerase chain reaction
Details of drug treatment and assays were described in the supplemental Methods.
In vivo experiment
Details of in vivo experiments were available in the supplemental Methods. Briefly, MM.1S cells stably expressing luciferase (5 × 105 cells/mouse) were injected via tail vein or subcutaneously into irradiated (2.5 Gy) NOD/SCID IL-2γ null (NSG) mice. Beginning at day 2 or at day 14, the mice were treated with ABC294640 (50 mg/kg, intraperitoneally) daily for ∼30 days. Tumor growth was followed by bioluminescence imaging at the time-points indicated.
Statistical analysis
Details of statistical analysis are available in the supplemental Methods.
Results
SK2 is overexpressed in myeloma cells
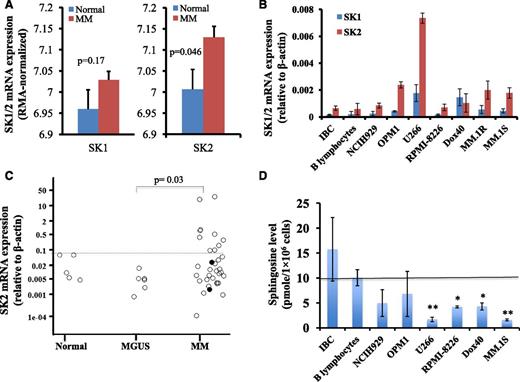
To determine the potential utility of targeting SKs for the treatment of MM, we measured the gene expression levels of SK1 and SK2 in myeloma cells. We measured SK1 and SK2 messenger RNA (mRNA) expression in a publicly available myeloma microarray dataset, in myeloma cell lines, and in freshly isolated human BM CD138+ myeloma cells (Figure 1). We downloaded the GSE6477 Affymetrix microarray dataset originated by Mayo Clinic.39,40 This dataset contained microarray mRNA gene profile on purified plasma cells isolated from normal control subjects (n = 15) or newly diagnosed MM patients (n = 73). We generated the robust multi-array average (RMA) normalized gene expression data for SK1 and SK2, and compared their expression levels between normal subjects and newly diagnosed MM patients. As shown in Figure 1A, SK2 expression was increased in MM patients compared with normal subjects (P = .046), whereas there was no significant difference in SK1 expression level in plasma cells between MM patients and normal subjects.
SK2 was overexpressed in myeloma cells. (A) SK1 and SK2 gene expression in GSE6477 Affymetrix microarray dataset. Publicly available Affymetrix microarray data set GSE6477 was downloaded and the RMA normalized gene expression data were generated. The SK1 and SK2 expression levels between plasma cells from normal subjects (blue bar; n = 15) and purified CD138+ cells from newly diagnosed MM (red bar; n = 73) were compared. (B) SK1 and SK2 expression in myeloma cell lines and B-cell lines. RNA was extracted from 2 B-cell lines (EBV-immortalized B cells (IBC) and American Type Culture Collection (ATCC) CCL-156 B-lymphocytes) and 7 MM cell lines (NCI-H929, OPM1, U266, RPMI-8226, RPMI-8226-Dox40, MM.1R, and MM.1S) and RT-PCR was performed for SK1 and SK2. Relative SK1 and SK2 mRNA expression with respect to β-actin was shown (mean ± standard error of the mean of 3 separate sets of experiments). (C) SK2 expression in primary human BM CD138+ cells. Primary human CD138+ cells were isolated using CD138 enrichment kit from the BM aspirates of normal subjects (n = 5), monoclonal gammopathy of undetermined significance patients (n = 6), and myeloma patients (n = 34). SK2 gene expression was normalized against β-actin control. (Each dot represented 1 individual patient and the two solid circles represented amyloidosis patients). (D) Sphingosine level in myeloma cell lines and B-cell lines. Sphingosine was measured by high performance liquid chromatography (HPLC) in freshly prepared Epstein-Barr virus (EBV)-immortalized B cells, ATCC B-lymphocytes, and 6 MM cell lines (NCI-H929, OPM1, U266, RPMI-8226, RPMI-8226-Dox40, and MM.1S). Data represented the sphingosine concentration (pmol/1 × 106 cells) (mean ± standard error of the mean of 1 of 4 separate sets of experiments) (*P < .05; **P < .01).
SK2 was overexpressed in myeloma cells. (A) SK1 and SK2 gene expression in GSE6477 Affymetrix microarray dataset. Publicly available Affymetrix microarray data set GSE6477 was downloaded and the RMA normalized gene expression data were generated. The SK1 and SK2 expression levels between plasma cells from normal subjects (blue bar; n = 15) and purified CD138+ cells from newly diagnosed MM (red bar; n = 73) were compared. (B) SK1 and SK2 expression in myeloma cell lines and B-cell lines. RNA was extracted from 2 B-cell lines (EBV-immortalized B cells (IBC) and American Type Culture Collection (ATCC) CCL-156 B-lymphocytes) and 7 MM cell lines (NCI-H929, OPM1, U266, RPMI-8226, RPMI-8226-Dox40, MM.1R, and MM.1S) and RT-PCR was performed for SK1 and SK2. Relative SK1 and SK2 mRNA expression with respect to β-actin was shown (mean ± standard error of the mean of 3 separate sets of experiments). (C) SK2 expression in primary human BM CD138+ cells. Primary human CD138+ cells were isolated using CD138 enrichment kit from the BM aspirates of normal subjects (n = 5), monoclonal gammopathy of undetermined significance patients (n = 6), and myeloma patients (n = 34). SK2 gene expression was normalized against β-actin control. (Each dot represented 1 individual patient and the two solid circles represented amyloidosis patients). (D) Sphingosine level in myeloma cell lines and B-cell lines. Sphingosine was measured by high performance liquid chromatography (HPLC) in freshly prepared Epstein-Barr virus (EBV)-immortalized B cells, ATCC B-lymphocytes, and 6 MM cell lines (NCI-H929, OPM1, U266, RPMI-8226, RPMI-8226-Dox40, and MM.1S). Data represented the sphingosine concentration (pmol/1 × 106 cells) (mean ± standard error of the mean of 1 of 4 separate sets of experiments) (*P < .05; **P < .01).
SK1 and SK2 mRNA expression levels in two B-cell lines (EBV-immortalized B-cell line and ATCC B lymphocyte cell line) and 7 myeloma cell lines (NCI-H929, OPM1, U266, RPMI-8226, RPMI-8226-Dox40, MM.1R, and MM.1S) were measured by quantitative reverse transcription-polymerase chain reaction (RT-PCR). As shown in Figure 1B, the mRNA expression level of SK2 was higher than that of SK1 in all tested myeloma cell lines, except RPMI-8226-Dox40 cells. Furthermore, the mRNA expression level of SK2 was higher in all 7 myeloma cell lines than that in the 2 B-cell lines.
We also determined the SK1 and SK2 mRNA gene expression in freshly isolated primary human BM CD138+ MM specimens. CD138+ plasma cells were isolated from the BM aspirates of normal controls, monoclonal gammopathy of undetermined significance patients, or MM patients including amyloidosis patients. No difference in SK1 mRNA expression was observed between these 3 populations of patients (data not shown). Interestingly, SK2 gene expression was increased in the CD138+ cells in 10 of 34 (29%) MM patients (Figure 1C). We performed additional subset analyses to determine whether SK2 mRNA expression correlated with myeloma disease stage, cytogenetic profile, M protein level, or BM plasma cell number. No correlation was observed with these subset analyses (data not shown).
SKs catalyze the phosphorylation of sphingosine to S1P and sphingosine derives from ceramide. To determine if the overexpression of SK2 in myeloma cells affects the levels of ceramides, sphingosine, and S1P, mass spectrometry measurement of 14 different ceramides, sphingosine, and S1P was performed in 2 B-cell lines and 6 MM cell lines. The levels of ceramides and S1P varied highly among MM cell lines and between B-cell lines and MM-cell lines (data not shown). Interestingly, the sphingosine level was lower in the MM cells than that in the B cells, and the difference was statistically significant for 4 of 6 MM cell lines we tested (Figure 1D). The decrease in the level of sphingosine in MM is consistent with increased sphingosine kinase gene expression.
SK2-specific shRNA inhibits myeloma proliferation and induces caspase 3-mediated cell death
To determine the roles of SK2 in MM cell survival and proliferation, we used specific shRNA to knockdown SK2 expression in MM cells. Lentiviral vector–expressing SK2-specific shRNA or control shRNA was constructed and used to transduce MM cell lines. Both SK2-specific shRNA and control shRNA effectively transduced MM cell lines as demonstrated by the high level of Discosoma red fluorescent protein (DsRFP) expression (Figure 2A). SK2-specific shRNA decreased SK2 mRNA expression by ∼80% (Figure 2B). SK2-specific shRNA effectively inhibited myeloma cell proliferation as measured by MTT assay (Figure 2C). To further determine the effect of SK2 on myeloma cell proliferation, we transduced OPM1 myeloma cells with SK2-specific shRNA or control shRNA. Then we labeled the cells with CellTrace Violet Cell Proliferation dye and measured dye fluorescence intensity 7 days later (Figure 2D). With cell division, the dye is diluted and the fluorescence intensity is reduced. As shown in Figure 2D, compared with control shRNA, SK2-specific RNA inhibited myeloma cell proliferation and division. In addition, we found that SK2-specific shRNA activated caspase 3 (Figure 2E). These data suggested that SK2 plays an important role in both cell proliferation and survival of myeloma cells, and thus provides a therapeutic target for the treatment of MM.
SK2-specific shRNA inhibited cell proliferation and induced caspase 3 activation in myeloma cells. OPM1 cells were transduced with lentiviruses expressing SK2-shRNA-DsRFP or control shRNA-DsRFP for 4 hours. The cells were then washed and grew in regular culture medium for an additional 48 hours. (A) Fluorescent microscopy image showing DsRFP expression. (B) Expression of SK2 mRNA in SK2-shRNA- or control shRNA-transduced OPM1 cells. (C) Cell proliferation by MTT assay. Cell proliferation was measured using MTT assay at 0 hours and 48 hours after transduction. (D) Cell proliferation by flow cytometry. OPM1 cells transduced with SK2-shRNA or control shRNA were stained with CellTrace Violet Cell Proliferation dye and allowed to proliferate for 7 days. The dye fluorescence intensity was measured by flow cytometry. (E) Activation of caspase 3. OPM1 cells were transduced with SK2-shRNA viruses or control shRNA viruses. Forty-eight hours later, the cells were stained with Live/Dead Fixable cell dye, then fixed and permeabilized, and stained with caspase-3 antibody. Caspase 3 intensity was gated on the live cell population. Data were representative of 4 separate experiments.
SK2-specific shRNA inhibited cell proliferation and induced caspase 3 activation in myeloma cells. OPM1 cells were transduced with lentiviruses expressing SK2-shRNA-DsRFP or control shRNA-DsRFP for 4 hours. The cells were then washed and grew in regular culture medium for an additional 48 hours. (A) Fluorescent microscopy image showing DsRFP expression. (B) Expression of SK2 mRNA in SK2-shRNA- or control shRNA-transduced OPM1 cells. (C) Cell proliferation by MTT assay. Cell proliferation was measured using MTT assay at 0 hours and 48 hours after transduction. (D) Cell proliferation by flow cytometry. OPM1 cells transduced with SK2-shRNA or control shRNA were stained with CellTrace Violet Cell Proliferation dye and allowed to proliferate for 7 days. The dye fluorescence intensity was measured by flow cytometry. (E) Activation of caspase 3. OPM1 cells were transduced with SK2-shRNA viruses or control shRNA viruses. Forty-eight hours later, the cells were stained with Live/Dead Fixable cell dye, then fixed and permeabilized, and stained with caspase-3 antibody. Caspase 3 intensity was gated on the live cell population. Data were representative of 4 separate experiments.
SK2-specific inhibitor (ABC294640) inhibits myeloma growth in vitro
Next, we tested the effectiveness of the SK2-selective inhibitor (ABC294640) in killing myeloma cells in vitro. ABC294640 is the most advanced, nonlipid-based oral SK2 inhibitor and shows no inhibition for SK1 or panel of protein kinases.41 ABC294640 is currently undergoing single agent phase I/II clinical trial at our institute for solid tumors. We treated 6 different MM cell lines with various concentrations of ABC294640 and found that ABC294640 inhibited myeloma cell growth with a median inhibition concentration (IC50) of ∼30 μM, including steroid resistant MM.1R cells (Figure 3A). Among the 6 MM cell lines we tested, OPM1 appears to be the most sensitive cell line to ABC294640 treatment, whereas U266 is relatively resistant. ABC294640 inhibited myeloma cell growth as early as 16 hours after exposure (Figure 3B).
ABC294640 inhibited cell proliferation and induced apoptosis in MM cells. (A) Dose-dependent inhibition of cell proliferation by ABC294640. Six different MM cell lines were treated with various concentrations of ABC294640 for 48 hours and cell proliferation was measured by MTT assay (mean ± standard error of the mean [SEM] of 1 of 3 separate sets of experiments). (B) Time course of proliferation inhibition by ABC294640. Six different MM cells lines were treated with 30 µM of ABC294640 or dimethylsulfoxide (DMSO) for various durations. Cell proliferation was analyzed by MTT assay at the time-points indicated (mean ± SEM of 1 of 3 separate sets of experiments). (C) Cytotoxic effects of ABC294640 on MM cells. OPM1 cells were treated with 30 µM of ABC294640 or DMSO and total live cells were quantified at the time-points indicated (mean ± SEM of 3 separate sets of experiments). (D) Increased Annexin V+ cells by ABC294640. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 16 hours and cells were stained with Annexin V and 7-amino-actinomycin D (AAD) and analyzed by flow cytometry. Data shown were representative of 3 separate sets of experiments. (E) Caspase 3 activation. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 16 hours and cells were then fixed, permeabilized, and stained with caspase3 antibody. (F) Caspase 9 activation and PAPR cleavage. OPM1 cells were treated with 30 µM of ABC294640 (indicated as [A]) or DMSO control (indicated as [D]) for 3, 6, 9, and 12 hours, and analyzed for PARP and cleaved PARP, full length caspase-9, and cleaved caspase-9 by western blot analysis. β-actin was used as a loading control (data were representative of 3 separate sets of experiments). (G) Inhibition of primary human CD138+ myeloma cells by ABC294640. Primary human CD138+ cells were freshly isolated using CD138 enrichment kit from the BM aspirates of myeloma patients and cultured in triplicate at 1 × 104 cells in 100 μL of RPMI1640 medium supplemented with 2 mM Glutamax and 10% fetal calf serum containing DMSO control or various concentrations of ABC294640 for 24 hours at 37°C under 5% CO2. Cell proliferation was then measured by MTT assay. OPM1 cells were similarly treated for comparison (n = 3; Sample ID #7244, #7452, and #7476).
ABC294640 inhibited cell proliferation and induced apoptosis in MM cells. (A) Dose-dependent inhibition of cell proliferation by ABC294640. Six different MM cell lines were treated with various concentrations of ABC294640 for 48 hours and cell proliferation was measured by MTT assay (mean ± standard error of the mean [SEM] of 1 of 3 separate sets of experiments). (B) Time course of proliferation inhibition by ABC294640. Six different MM cells lines were treated with 30 µM of ABC294640 or dimethylsulfoxide (DMSO) for various durations. Cell proliferation was analyzed by MTT assay at the time-points indicated (mean ± SEM of 1 of 3 separate sets of experiments). (C) Cytotoxic effects of ABC294640 on MM cells. OPM1 cells were treated with 30 µM of ABC294640 or DMSO and total live cells were quantified at the time-points indicated (mean ± SEM of 3 separate sets of experiments). (D) Increased Annexin V+ cells by ABC294640. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 16 hours and cells were stained with Annexin V and 7-amino-actinomycin D (AAD) and analyzed by flow cytometry. Data shown were representative of 3 separate sets of experiments. (E) Caspase 3 activation. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 16 hours and cells were then fixed, permeabilized, and stained with caspase3 antibody. (F) Caspase 9 activation and PAPR cleavage. OPM1 cells were treated with 30 µM of ABC294640 (indicated as [A]) or DMSO control (indicated as [D]) for 3, 6, 9, and 12 hours, and analyzed for PARP and cleaved PARP, full length caspase-9, and cleaved caspase-9 by western blot analysis. β-actin was used as a loading control (data were representative of 3 separate sets of experiments). (G) Inhibition of primary human CD138+ myeloma cells by ABC294640. Primary human CD138+ cells were freshly isolated using CD138 enrichment kit from the BM aspirates of myeloma patients and cultured in triplicate at 1 × 104 cells in 100 μL of RPMI1640 medium supplemented with 2 mM Glutamax and 10% fetal calf serum containing DMSO control or various concentrations of ABC294640 for 24 hours at 37°C under 5% CO2. Cell proliferation was then measured by MTT assay. OPM1 cells were similarly treated for comparison (n = 3; Sample ID #7244, #7452, and #7476).
To determine if ABC294640 induces cytotoxic or cytostatic effects on MM cells, we cultured MM cells with 30 μM of ABC294640, and then we quantified live cell numbers over time. As shown in Figure 3C, ABC294640 exhibited cytotoxic effects on the majority (7 of 8) MM cell lines we tested, except for U266 cells. Furthermore, we found that ABC294640 treatment induced apoptotic cell death in myeloma cells, as demonstrated by Annexin V staining (Figure 3D), caspase 3 activation (Figure 3E), caspase 9 activation, and poly(ADP-ribose) (PAR) polymerase (PARP) cleavage (Figure 3F). ABC294640 induced growth arrest, but no apoptosis in U266 cells (data not shown).
We also tested the antitumor effects of ABC294640 on primary human CD138+ myeloma cells. Human CD138+ myeloma cells were freshly isolated from myeloma patients' BM aspirates and treated with various concentrations of ABC294640. Cell proliferation was then measured by MTT assay. ABC294640 inhibited primary human myeloma cells with the same efficacy as with the most sensitive myeloma cell lines (OPM1) (Figure 3G), demonstrating the potential clinical utility of ABC294640 in the treatment of MM.
The effects of SK1 inhibitor (SK1-II), myriocin (a natural product inhibitor of serine palmitoyltransferase), and FTY720 (a S1P receptor antagonist) on myeloma cell proliferation were investigated (supplemental Data). Consistent with previous reports by others,25 FTY720 inhibited myeloma cell growth (supplemental Figure 1A). No inhibitory effects of myriocin or SK1 inhibitor on myeloma cells were observed (supplemental Figure 1B-C). Again, these data support the important role of SK2 in myeloma cell proliferation and survival.
ABC294640 upregulates Noxa expression and promotes proteasome degradation of Mcl-1
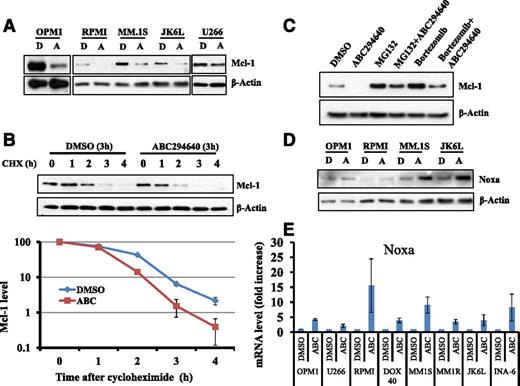
We performed extensive mechanistic studies to understand the pathways through which SK2 inhibition induces myeloma cell death. Extensive studies have demonstrated an essential role of myeloid cell leukemia 1 (Mcl-1) in the survival of human myeloma cells.42,43 Mcl-1 is overexpressed in more than half of newly diagnosed MM patients and in ∼81% of relapsed MM patients.44 Furthermore, recent studies suggested that SK1 plays a key role in Mcl-1 expression induced by IL-6.26 Therefore, we examined if ABC294640 could affect the expression of Mcl-1 in MM. As shown in Figure 4A, ABC294640 treatment led to downregulation of Mcl-1 protein expression in MM cells, with the largest reduction occurring in OPM1 cells, which is also the most sensitive cell line for inhibition of proliferation by ABC294640. In addition, ABC294640 caused minimal changes in Mcl-1 expression in U266 cells.
ABC294640 enhanced Mcl-1 proteasome degradation and increased Noxa expression. (A) ABC294640 downregulated Mcl-1 protein expression. MM cells (OPM1, RPMI-8226, MM.1S, JK6L, and U266) were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours, and whole cell lysates were prepared and analyzed for Mcl-1 expression by western blot analysis. β-actin was used as the loading control. Data were representative of 3 separate sets of experiments. (B) A294640 increased Mcl-1 protein degradation. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 3 hours and then cycloheximide (100 µg/mL) was added. Cells were collected at each hour after cycloheximide treatment and whole cell lysate was prepared and analyzed for Mcl-1 expression by western blot analysis. β-actin was used as the loading control. The graph represents the quantification of western blots. The western blots were quantified using ImageJ. Data were representative of 2 separate sets of experiments. (C) Proteasome inhibitor (MG132 and bortezomib) prevented the degradation of Mcl-1 by ABC294640. OPM1 cells were treated with DMSO control buffer, proteasome inhibitor MG132 (1 µM), or bortezomib (50 nM) for 1 hour, followed by treatment with DMSO or 30 µM of ABC294640 for an additional 6 hours. Whole cell lysate was prepared and analyzed for Mcl-1 expression by western blot analysis. Data were representative of 2 separate sets of experiments. (D) ABC294640 increased Noxa protein expression. OPM1, RPMI8226, MM.1S, and JK6L were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours, and whole cell lysates were prepared and analyzed for Noxa expression by western blot analysis. (E) ABC294640 induced Noxa gene expression. Eight MM cell lines were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours and RNA was isolated and analyzed for Noxa gene expression by RT-PCR. Gene expression was normalized against β-actin internal control. Graphs represented the fold change of Noxa mRNA in ABC294640-treated MM cells lines compared with DMSO-treated cells. Data shown in the figure were representative of at least 2 separate sets of experiments.
ABC294640 enhanced Mcl-1 proteasome degradation and increased Noxa expression. (A) ABC294640 downregulated Mcl-1 protein expression. MM cells (OPM1, RPMI-8226, MM.1S, JK6L, and U266) were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours, and whole cell lysates were prepared and analyzed for Mcl-1 expression by western blot analysis. β-actin was used as the loading control. Data were representative of 3 separate sets of experiments. (B) A294640 increased Mcl-1 protein degradation. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 3 hours and then cycloheximide (100 µg/mL) was added. Cells were collected at each hour after cycloheximide treatment and whole cell lysate was prepared and analyzed for Mcl-1 expression by western blot analysis. β-actin was used as the loading control. The graph represents the quantification of western blots. The western blots were quantified using ImageJ. Data were representative of 2 separate sets of experiments. (C) Proteasome inhibitor (MG132 and bortezomib) prevented the degradation of Mcl-1 by ABC294640. OPM1 cells were treated with DMSO control buffer, proteasome inhibitor MG132 (1 µM), or bortezomib (50 nM) for 1 hour, followed by treatment with DMSO or 30 µM of ABC294640 for an additional 6 hours. Whole cell lysate was prepared and analyzed for Mcl-1 expression by western blot analysis. Data were representative of 2 separate sets of experiments. (D) ABC294640 increased Noxa protein expression. OPM1, RPMI8226, MM.1S, and JK6L were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours, and whole cell lysates were prepared and analyzed for Noxa expression by western blot analysis. (E) ABC294640 induced Noxa gene expression. Eight MM cell lines were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours and RNA was isolated and analyzed for Noxa gene expression by RT-PCR. Gene expression was normalized against β-actin internal control. Graphs represented the fold change of Noxa mRNA in ABC294640-treated MM cells lines compared with DMSO-treated cells. Data shown in the figure were representative of at least 2 separate sets of experiments.
Protein expression is controlled by the rate of biosynthesis and degradation. Thus, we first determined if ABC294640 affected Mcl-1 gene transcription. MM cell lines were treated with ABC294640 for 16 hours and Mcl-1 mRNA was quantified by RT-PCR. In addition, OPM1 cells were stably transduced with SK2-specific shRNA or control shRNA, and Mcl-1 mRNA was measured. Inhibition of SK2 by either ABC294640 or shRNA did not affect Mcl-1 gene transcription (supplemental Figure 2), suggesting that ABC294640 downregulated Mcl-1 expression at the posttranscription level.
Next, we tested if ABC294640 would increase the rate of Mcl-1 degradation. To this end, we treated OPM1 cells with DMSO or ABC294640 for 3 hours, and then added cycloheximide to inhibit new protein synthesis. Mcl-1 protein levels were measured by immunoblot analysis every hour for 4 hours. As shown in Figure 4B, ABC294640 treatment significantly increased Mcl-1 degradation.
Proteasome degradation plays an important role in regulating protein stability. Thus, we tested if ABC294640 treatment promoted Mcl-1 degradation in a proteasome-dependent manner. OPM1 cells were treated with ABC294640 alone, proteasome inhibitor (either MG132 or bortezomib) alone or in combination of ABC294640 with proteasome inhibitor. MG132 and bortezomib partially, but reproducibly protected Mcl-1 from degradation induced by ABC294640 treatment (Figure 4C). These data suggested that ABC294640 at least in part increased Mcl-1 proteasome degradation.
Noxa is a proapoptotic, B-cell lymphoma 2 (Bcl-2) homolog (BH) 3-only member of the Bcl-2 family. Treatment with bortezomib or arsenic trioxide induced upregulation of Noxa, while downregulating Mcl-1.45,46 These studies and the study by Alves et al47 suggested that Noxa and Mcl-1 form a molecule complex, and the Noxa/Mcl-1 ratio plays an important role in regulating apoptosis. Thus, we examined the effects of ABC294640 on Noxa expression. ABC294640 treatment increased Noxa protein expression levels by 50% to twofold (Figure 4D). In addition, ABC294640 upregulated Noxa mRNA expression in all 8 MM cell lines we tested by at least fivefold (Figure 4E). These data suggested that ABC294640 treatment shifted the Noxa/Mcl-1 apoptosis rheostat toward favoring cell death.
ABC294640 downregulates pS6 and promotes proteasome degradation of c-Myc
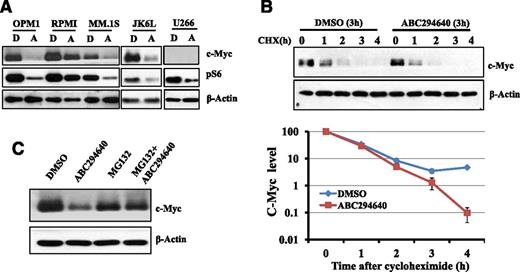
An important oncogene is c-Myc, which is dysregulated in postgerminal center malignancies including MM. Translocation involving c-MYC was found in 19 of 20 MM cell lines and in approximately 50% of advanced primary MM tumors.48 MM cells are addicted to c-Myc activity and c-Myc is indispensable in myeloma development.49 Furthermore, constitutive activation of PI3K/AKT/mammalian target of rapamycin (mTOR) pathway is a common event in MM pathogenesis and contributes to MM proliferation and survival.50 Our unpublished data indicated that the downstream of mTOR pathway (ie, pS6) was highly upregulated in nearly all myeloma patients’ bone marrow samples. Given the importance of c-Myc and pS6 in MM cell survival, we examined the effects of ABC294640 on the expression of c-Myc and pS6 in MM cells. ABC294640 treatment significantly downregulated the expression of c-Myc and pS6 (Figure 5A). U266 has no detectable c-Myc expression by western blot analysis.
ABC294640 enhanced c-Myc proteasome degradation. (A) ABC294640 downregulated c-Myc and pS6 protein expression. MM cells (OPM1, RPMI-8226, MM.1S, JK6L, and U266) were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours, and whole cell lysates were prepared and analyzed for c-Myc and pS6 expression by western blot analysis. β-actin was used as the loading control. (B) ABC294640 increased c-Myc protein degradation. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 3 hours and then cyclohexamide (100 µg/mL) was added. Cells were collected at each hour after cyclohexamide treatment, and whole cell lysate was prepared and analyzed for c-Myc expression by western blot analysis. β-actin was used as the loading control. The graph represents the quantification of western blots using ImageJ. (C) Proteasome inhibitor (MG132) prevented the degradation of c-Myc by ABC294640. OPM1 cells were treated with DMSO control buffer or MG132 (1 µM) for 1 hour, followed by treatment with DMSO or 30 µM of ABC294640 for an additional 6 hours. Whole cell lysate was prepared and analyzed for c-Myc expression by western blot analysis. Data shown in the figure were representative of at least 2 separate sets of experiments.
ABC294640 enhanced c-Myc proteasome degradation. (A) ABC294640 downregulated c-Myc and pS6 protein expression. MM cells (OPM1, RPMI-8226, MM.1S, JK6L, and U266) were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours, and whole cell lysates were prepared and analyzed for c-Myc and pS6 expression by western blot analysis. β-actin was used as the loading control. (B) ABC294640 increased c-Myc protein degradation. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 3 hours and then cyclohexamide (100 µg/mL) was added. Cells were collected at each hour after cyclohexamide treatment, and whole cell lysate was prepared and analyzed for c-Myc expression by western blot analysis. β-actin was used as the loading control. The graph represents the quantification of western blots using ImageJ. (C) Proteasome inhibitor (MG132) prevented the degradation of c-Myc by ABC294640. OPM1 cells were treated with DMSO control buffer or MG132 (1 µM) for 1 hour, followed by treatment with DMSO or 30 µM of ABC294640 for an additional 6 hours. Whole cell lysate was prepared and analyzed for c-Myc expression by western blot analysis. Data shown in the figure were representative of at least 2 separate sets of experiments.
We performed additional studies similar to those described with Mcl-1 to understand the mechanisms through which ABC294640 downregulated c-Myc expression. We found that ABC294640 or SK2-specific shRNA did not affect the rate of c-Myc gene transcription (supplemental Figure 3). The cycloheximide study suggested that ABC294640 increased c-Myc protein degradation (Figure 5B). MG132 protected c-Myc from ABC294640-induced c-Myc degradation, suggesting that ABC294640 enhances proteasome degradation of c-Myc (Figure 5C).
ABC294640 synergizes with Bcl-2 inhibitor in the killing of myeloma cells
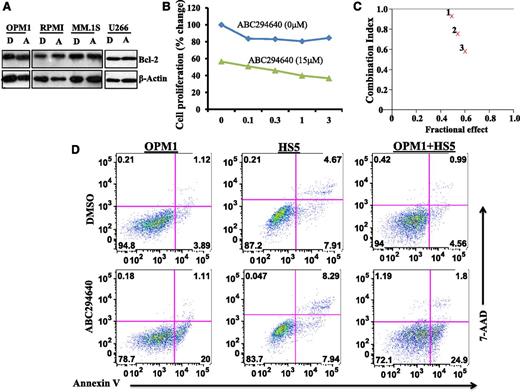
We reasoned that combined chemotherapy incorporating drugs with different mechanisms of action is likely to be more effective in killing myeloma cells and could overcome drug resistance. To this end, we examined the antimyeloma activity of ABC294640 in combination with other agents. We were particularly interested in combining ABC294640 with Bcl-2 inhibitor for the treatment of MM because ABC294640 per se did not affect Bcl-2 expression (Figure 6A). We treated MM cell lines with various concentrations of ABT-737 (a Bcl-2 inhibitor) in combination with 15 µM of ABC294640. Cell proliferation was then measured by MTT assay. Combining ABC294640 with ABT-737 led to greater inhibition of MM cell proliferation (Figure 6B). Additional ABC294640 concentrations were tested with ABT-737 and the combination index value was calculated. Fraction affected combination index (Fa-CI) plot analysis demonstrated synergism between ABC294640 and ABT-737 in inhibiting myeloma cell proliferation (Figure 6C).
ABC294640 acted synergistically with Bcl-2 inhibitor in inhibiting myeloma cell growth and induced myeloma cell apoptosis in the presence of BM stromal cells. (A) ABC294640 did not affect Bcl-2 expression. MM cells (OPM1, RPMI-8226, MM.1S, and U266) were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours, and whole cell lysates were prepared and analyzed for Bcl-2 expression by western blot analysis. (B) Combination of ABC294640 and ABT-737 led to enhanced inhibition of cell proliferation. OPM1 cells were treated with various concentrations of ABT-737 in the absence or presence of ABC294640 (15 µM) for 48 hours and cell proliferation was measured by MTT assay. (C) FA-CI plots showing the synergistic effect of ABC294640 and ABT-737. Fa-CI plots for OPM1 cells revealed a synergistic inhibitory effect for ABC294640 15 μM and ABT-737 at 0.1 µM (indicated as 1), 0.3 µM (indicated as 2), and 1 µM (indicated as 3). In the Fa-CI plot, the line (combination index = 1) indicate an additive reaction between the 2 substances. Values below this line imply synergism. (D) ABC294640 induced myeloma apoptosis in the presence of BM stromal cells. GFP-expressing OPM1 cells were cultured on the monolayer of HS5 BM stromal cells and were treated for 8 hours with 30 µM of ABC294640 or DMSO. The cells were stained with Annexin V and 7-amino-actinomycin D (AAD) and Annexin V+ apoptotic cells were gated on GFP-positive OPM1 cells.
ABC294640 acted synergistically with Bcl-2 inhibitor in inhibiting myeloma cell growth and induced myeloma cell apoptosis in the presence of BM stromal cells. (A) ABC294640 did not affect Bcl-2 expression. MM cells (OPM1, RPMI-8226, MM.1S, and U266) were treated with 30 µM of ABC294640 (A) or DMSO (D) for 16 hours, and whole cell lysates were prepared and analyzed for Bcl-2 expression by western blot analysis. (B) Combination of ABC294640 and ABT-737 led to enhanced inhibition of cell proliferation. OPM1 cells were treated with various concentrations of ABT-737 in the absence or presence of ABC294640 (15 µM) for 48 hours and cell proliferation was measured by MTT assay. (C) FA-CI plots showing the synergistic effect of ABC294640 and ABT-737. Fa-CI plots for OPM1 cells revealed a synergistic inhibitory effect for ABC294640 15 μM and ABT-737 at 0.1 µM (indicated as 1), 0.3 µM (indicated as 2), and 1 µM (indicated as 3). In the Fa-CI plot, the line (combination index = 1) indicate an additive reaction between the 2 substances. Values below this line imply synergism. (D) ABC294640 induced myeloma apoptosis in the presence of BM stromal cells. GFP-expressing OPM1 cells were cultured on the monolayer of HS5 BM stromal cells and were treated for 8 hours with 30 µM of ABC294640 or DMSO. The cells were stained with Annexin V and 7-amino-actinomycin D (AAD) and Annexin V+ apoptotic cells were gated on GFP-positive OPM1 cells.
ABC294640 induces myeloma cell apoptosis in the presence of BM stromal cells
BM stromal cells support the growth and survival of myeloma cells and confer them with drug resistance. We sought to test if ABC294640 could still effectively kill myeloma cells in the presence of BM stromal cells. We cocultured enhanced green fluorescent protein (eGFP)-expressing myeloma cells with HS5 BM stromal cells. We then added ABC294640 to the coculture system and measured Annexin V+ cells gated on the eGFP+ myeloma cells. ABC294640 did not induce apoptosis of HS5 BM stromal cells (Figure 6D). Interestingly, as shown in Figure 6D, the percentage of Annexin V+ myeloma cells after ABC294640 treatment was quite similar in OPM1 cells alone and in OPM1 cells cocultured with HS5 BM stromal cells. These data suggested that ABC294640 could induce OPM1 cell apoptosis, even in the presence of HS5 BM stromal cells.
ABC294640 suppresses MM tumor growth in mouse xenograft models
The in vivo antimyeloma activity of ABC294640 was assessed using mouse xenograft models. We transduced MM.1S myeloma cells with lentiviral vector encoding luciferase and generated the MM.1S cell line stably expressing luciferase. We performed 2 series of in vivo experiments. In the first series of experiments, the luciferase-expressing MM.1S cells were injected via tail vein (Figure 7A) or subcutaneously (Figure 7B) into sublethally irradiated NSG mice. Two days after the tumor injection, the mice were treated with ABC294640 (50 mg/kg daily intraperitoneally) or vehicle-control buffer for 30 days. In our second series of experiments, MM.1S cells were injected subcutaneously into irradiated (2.5 Gy) NSG mice. ABC294640 treatment was started 14 days later when bioluminescence imaging showed tumor engraftment, and the treatment continued daily for ∼1 month (Figure 7C). Tumor growth was monitored by bioluminescence imaging at the time points indicated. As shown in Figure 7A-C, ABC294640 effectively inhibited myeloma growth in vivo.
ABC294640 suppressed myeloma growth in vivo in mouse xenograft models. (A) ABC294640 inhibited myeloma growth in vivo in the intravenously administrated mouse xenograft model. NOD/SCID IL-2γ null (NSG) mice were sublethally irradiated (2.5 Gy) and injected via tail vein 0.5 × 106 MM.1S myeloma cells stably expressing luciferase. Two days later, the mice were divided randomly into 2 groups and treated with either ABC 294640 50 mg/kg intraperitoneally (A) or a control vehicle (C) (phosphate-buffered saline + 0.3% Tween-80) for 30 days. Every 10 days, the mice were imaged using the Perkin Elmer Ivis 200 imager and Live Image software. (B) ABC294640 inhibited myeloma growth in vivo in the subcutaneously inoculated mouse xenograft model. NSG mice were sublethally irradiated (2.5 Gy) and injected subcutaneously with 0.5 × 106 MM.1S myeloma cells stably expressing luciferase. Two days later, the mice were treated with either ABC294640 50 mg/kg intraperitoneally (mice indicated as “A”) or control vehicle (mouse “C”) for 30 days. Tumor growth was monitored by bioluminescence imaging. (C) ABC294640 inhibited myeloma growth in vivo. NSG mice were sublethally irradiated (2.5 Gy) and injected subcutaneously with 0.5 × 106 MM.1S myeloma cells stably expressing luciferase. Two weeks later, the mice were imaged using bioluminescence imaging and showed tumor engraftment (D0). The mice were then treated with ABC294640 50 mg/kg intraperitoneally or control daily for 27 days (from D0 to D27). Tumor growth was monitored by bioluminescence imaging at time-points indicated (up to 1 week after the discontinuation of injection [ie, D35]). (D) Schematic diagram of the mechanisms of action of ABC294640 in MM cells.
ABC294640 suppressed myeloma growth in vivo in mouse xenograft models. (A) ABC294640 inhibited myeloma growth in vivo in the intravenously administrated mouse xenograft model. NOD/SCID IL-2γ null (NSG) mice were sublethally irradiated (2.5 Gy) and injected via tail vein 0.5 × 106 MM.1S myeloma cells stably expressing luciferase. Two days later, the mice were divided randomly into 2 groups and treated with either ABC 294640 50 mg/kg intraperitoneally (A) or a control vehicle (C) (phosphate-buffered saline + 0.3% Tween-80) for 30 days. Every 10 days, the mice were imaged using the Perkin Elmer Ivis 200 imager and Live Image software. (B) ABC294640 inhibited myeloma growth in vivo in the subcutaneously inoculated mouse xenograft model. NSG mice were sublethally irradiated (2.5 Gy) and injected subcutaneously with 0.5 × 106 MM.1S myeloma cells stably expressing luciferase. Two days later, the mice were treated with either ABC294640 50 mg/kg intraperitoneally (mice indicated as “A”) or control vehicle (mouse “C”) for 30 days. Tumor growth was monitored by bioluminescence imaging. (C) ABC294640 inhibited myeloma growth in vivo. NSG mice were sublethally irradiated (2.5 Gy) and injected subcutaneously with 0.5 × 106 MM.1S myeloma cells stably expressing luciferase. Two weeks later, the mice were imaged using bioluminescence imaging and showed tumor engraftment (D0). The mice were then treated with ABC294640 50 mg/kg intraperitoneally or control daily for 27 days (from D0 to D27). Tumor growth was monitored by bioluminescence imaging at time-points indicated (up to 1 week after the discontinuation of injection [ie, D35]). (D) Schematic diagram of the mechanisms of action of ABC294640 in MM cells.
Discussion
In the current study, we found that SK2 was overexpressed in myeloma cells and 10 of 34 (∼29%) MM patients had upregulated SK2 expression (Figure 1C). Furthermore, knockdown of the SK2 gene with shRNA inhibited myeloma cell proliferation and induced cell death. These data demonstrated an important role of SK2 in myeloma pathogenesis. Using a SK2 specific inhibitor, we showed (for the first time) that SK2 provides a potential therapeutic target for the treatment of MM. In addition, we investigated the combinatorial antitumor effects of ABC294640 with other categories of antimyeloma agents. We found that ABC294640 acts synergistically with Bcl-2 inhibitor (ABT-737) in killing myeloma cells. This novel combination of ABC294640 with Bcl-2 inhibitor (Figure 7D) may be particularly important in the treatment of relapsed or refractory MM patients. This population of patients most likely has been exposed to and become resistant to currently available agents such as dexamethasone, immunomodulatory drugs, and proteasome inhibitors.
ABC29464041,51-58 is currently undergoing a single-agent phase I clinical trial at our institute in patients with advanced solid tumors; to date, 16 patients have been enrolled in this trial showing no drug-related toxicities and demonstrating an excellent correlation between mouse and dog PK parameters with human data. ABC294640 has shown good pharmacokinetics, oral bioavailability, and biodistribution. Plasma concentrations can reach >200 µM without hematologic or major organ toxicity, about sixfold to sevenfold higher than the IC50 we found with MM cell lines. The anti-myeloma activity of ABC294640, seen with freshly isolated primary human CD138+ cells and in our in vivo mouse xenograft models, further provides justification for testing ABC294640 in a clinical setting with refractory/relapsed myeloma patients.
Mechanistically, we found that inhibition of SK2 upregulated Noxa expression and enhanced the proteasome degradation of Mcl-1 and c-Myc (Figure 7D). In addition, ABC294640 was found to downregulate the pS6 expression level. It is suggested by this finding that SK2 may also play a role in the mTOR signaling pathway and affect cell translation. It is currently unknown whether ABC294640 affects a common upstream event that controls Mcl-1, c-Myc, and pS6 pathways or if it has diverse biological activities affecting multiple pathways simultaneously. It also remains to be determined which effect (ie, downregulation of Mcl-1, c-Myc, or pS6) contributes to a larger extent to the killing of myeloma cells by ABC294640. We have found that ABC294640 induced apoptosis in OPM1 cells, but failed to do so in U266 cells. Interestingly, U266 cells do not express detectable level of c-Myc (Figure 5A) and show minimal change in Mcl-1 expression after ABC294640 treatment (Figure 4A). On the other hand, ABC294640 reduced pS6 expression in both U266 cells and OPM1 cells. These data suggested that among these 3 molecules, Mcl-1 might play a major role in mediating ABC294640-induced apoptosis. We are currently undertaking experiments to overexpress c-Myc in U266 cells or transduce c-Myc dominant negative construct into OPM1 cells to further dissect the role of c-Myc in ABC294640-mediated apoptosis in MM cells.
Treatment of proteasome inhibitors consistently, although partially reversed ABC294640-mediated downregulation of Mcl-1 protein expression (Figure 4C), suggesting that ABC294640 promotes Mcl-1 degradation at least in part via proteasome degradation pathway. It is also possible that ABC294640 may affect other protein degradation pathways including lysosomal proteolysis-mediated protein degradation or protein translation as suggested by the downregulation of pS6, which correlates with cell translation activities.
The relative roles of SK1 and SK2 in tumor biology have been of great interest to many investigators, and these were a central issue in the selection of ABC294640 for our study. Many studies have suggested different biological roles of the 2 SK isozymes.5,17,32-35 Most studies have focused on SK1 because it is the predominant isozyme in most cells, and is upregulated in many cancers. The latter response is likely due to hypoxia because SK1 is hypoxia inducible factor-regulated,59-62 making it difficult to discern whether overexpression is required to drive tumor growth or is a consequence of tumor growth. Target validation studies using complementary DNA transfection or RNA interference are inconsistent in ascribing dominance to either isozyme. For example, overexpression of SK1 has been shown to be oncogenic,63,64 whereas transfection with SK2 was originally reported to inhibit cell growth and induce apoptosis.65 However, these effects of SK2 are not dependent on its catalytic activity and may be mediated by its BH3 domain.65 Consistent with this are the observations that physiological levels of SK2 do not inhibit DNA synthesis.66 Published studies have also shown conflicting results on SK1 and SK2 expression. For instance, 1 study found that epidermal growth factor induced the expression of SK1, but not SK2 in MCF-7 cells,67 whereas others reported that epidermal growth factor activated SK2 in MDA-MB-453 cells.68 Knockdown of SK2 had more profound effects than SK1 knockdown in inhibiting glioblastoma cell growth.69 Our results with SK1 inhibitor did not reveal any effects of SK1 inhibitor at a concentration up to 60 µM on MM cell growth (supplemental Figure 1). Additional work is needed to refine our knowledge about SK1 vs SK2 biology in MM.
The exact role of SK2 in MM pathogenesis remains to be determined. SK2 deficient MCF-7 breast cancer showed delayed tumor growth in vivo in a mouse xenograft model, but the authors attributed this delay in tumor development to polarization of tumor-associated macrophages.70 It is unclear if the overexpression of SK2 in myeloma cells serves as a driving event to initiate the development of MM or if it merely reflects phenotypical changes due to other oncogene aberrations. There are no reports in the literature suggesting an increased incidence of myeloma in SK2 transgenic mice. We are currently planning to crossbreed the IL-6/c-MYC double transgenic myeloma mice with SK2 knockout mice and determine the incidence and severity of myeloma in the IL-6/c-Myc double transgenic/SK2 knockout mice. These studies will help us further define the role of SK2 in MM development.
In summary, SK2 is aberrantly upregulated in MM cells, and inhibition of SK2 suppresses myeloma growth in vitro and in vivo. Inhibition of SK2 affects several pathways critical to myeloma cell survival and proliferation including Mcl-1/Noxa, c-Myc, and pS6. Our study provides important preclinical data for testing the SK2 inhibitor (ABC294640) in clinical settings.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by MUSC Hollings Cancer Center Startup Fund, Hollings Cancer Center American Cancer Society-Institutional Research Grant, American Society of Clinical Oncology Conquer Cancer Foundation Career Development Award, National Institutes of Health (National Heart, Lung, and Blood Institute grant K08HL103780 and National Cancer Institute grant P30CA138313).
Authorship
Contribution: J.K.V., N.A., and Y.K. initiated the research, developed the concept of the paper, designed the study, and analyzed and interpreted the data; H.C. and J.H.S. performed part of the experiments; R.S., L.C., W.C., K.G., T.M., and Y.K. obtained patient consent and primary human BM samples; E.G.-M. and Z.W. assisted in biostatistical analyses; C.S. and B.O. provided reagents and helped design the study; and J.K.V. and Y.K. wrote the manuscript.
Conflict-of-interest disclosure: C.S. is the president and CEO of Apogee Biotechnology Corporation. The remaining authors declare no competing financial interests.
Correspondence: Yubin Kang, Division of Hematologic Malignancies and Cellular Therapy, Duke University, DUMC 3961, Durham, NC 27710; e-mail: yubin.kang@duke.edu.
References
Author notes
J.K.V. and N.A. contributed equally to this study.


![Figure 3. ABC294640 inhibited cell proliferation and induced apoptosis in MM cells. (A) Dose-dependent inhibition of cell proliferation by ABC294640. Six different MM cell lines were treated with various concentrations of ABC294640 for 48 hours and cell proliferation was measured by MTT assay (mean ± standard error of the mean [SEM] of 1 of 3 separate sets of experiments). (B) Time course of proliferation inhibition by ABC294640. Six different MM cells lines were treated with 30 µM of ABC294640 or dimethylsulfoxide (DMSO) for various durations. Cell proliferation was analyzed by MTT assay at the time-points indicated (mean ± SEM of 1 of 3 separate sets of experiments). (C) Cytotoxic effects of ABC294640 on MM cells. OPM1 cells were treated with 30 µM of ABC294640 or DMSO and total live cells were quantified at the time-points indicated (mean ± SEM of 3 separate sets of experiments). (D) Increased Annexin V+ cells by ABC294640. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 16 hours and cells were stained with Annexin V and 7-amino-actinomycin D (AAD) and analyzed by flow cytometry. Data shown were representative of 3 separate sets of experiments. (E) Caspase 3 activation. OPM1 cells were treated with 30 µM of ABC294640 or DMSO for 16 hours and cells were then fixed, permeabilized, and stained with caspase3 antibody. (F) Caspase 9 activation and PAPR cleavage. OPM1 cells were treated with 30 µM of ABC294640 (indicated as [A]) or DMSO control (indicated as [D]) for 3, 6, 9, and 12 hours, and analyzed for PARP and cleaved PARP, full length caspase-9, and cleaved caspase-9 by western blot analysis. β-actin was used as a loading control (data were representative of 3 separate sets of experiments). (G) Inhibition of primary human CD138+ myeloma cells by ABC294640. Primary human CD138+ cells were freshly isolated using CD138 enrichment kit from the BM aspirates of myeloma patients and cultured in triplicate at 1 × 104 cells in 100 μL of RPMI1640 medium supplemented with 2 mM Glutamax and 10% fetal calf serum containing DMSO control or various concentrations of ABC294640 for 24 hours at 37°C under 5% CO2. Cell proliferation was then measured by MTT assay. OPM1 cells were similarly treated for comparison (n = 3; Sample ID #7244, #7452, and #7476).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/12/10.1182_blood-2014-03-559385/4/m_1915f3.jpeg?Expires=1769302957&Signature=sS99tN4NSkTioB3lcAhPa6HuWsvsr4Jk~UgaHsTsWpDc6enfqjdqJZ~dh9IuakwFNSUefdZKGo3E4i2MTKOhPrDFoj4d3OEq~aArY4yiliCM6WDdtyLXW42nLwxxO4Qc-UIL6p0f62ezg4ti-wfsIJJsRRDlI7JHBI5kNuvT4gQs71vTNT5RqLZwpMcvM-8dESOgM3OVzUB455E1n~DmdZEmQKT~LB-8mOjcFifbfV0xuf62jxQCr27zAKNu60uS4VRODEfDwnwQ83pDO5vMJI2RThkZK5TwzfltF~cMUgtyiAXgwXVcaDABGY1PKRB9FzFbAoEFuRCy6ZJ-pz4TbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. ABC294640 suppressed myeloma growth in vivo in mouse xenograft models. (A) ABC294640 inhibited myeloma growth in vivo in the intravenously administrated mouse xenograft model. NOD/SCID IL-2γ null (NSG) mice were sublethally irradiated (2.5 Gy) and injected via tail vein 0.5 × 106 MM.1S myeloma cells stably expressing luciferase. Two days later, the mice were divided randomly into 2 groups and treated with either ABC 294640 50 mg/kg intraperitoneally (A) or a control vehicle (C) (phosphate-buffered saline + 0.3% Tween-80) for 30 days. Every 10 days, the mice were imaged using the Perkin Elmer Ivis 200 imager and Live Image software. (B) ABC294640 inhibited myeloma growth in vivo in the subcutaneously inoculated mouse xenograft model. NSG mice were sublethally irradiated (2.5 Gy) and injected subcutaneously with 0.5 × 106 MM.1S myeloma cells stably expressing luciferase. Two days later, the mice were treated with either ABC294640 50 mg/kg intraperitoneally (mice indicated as “A”) or control vehicle (mouse “C”) for 30 days. Tumor growth was monitored by bioluminescence imaging. (C) ABC294640 inhibited myeloma growth in vivo. NSG mice were sublethally irradiated (2.5 Gy) and injected subcutaneously with 0.5 × 106 MM.1S myeloma cells stably expressing luciferase. Two weeks later, the mice were imaged using bioluminescence imaging and showed tumor engraftment (D0). The mice were then treated with ABC294640 50 mg/kg intraperitoneally or control daily for 27 days (from D0 to D27). Tumor growth was monitored by bioluminescence imaging at time-points indicated (up to 1 week after the discontinuation of injection [ie, D35]). (D) Schematic diagram of the mechanisms of action of ABC294640 in MM cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/12/10.1182_blood-2014-03-559385/4/m_1915f7.jpeg?Expires=1769302957&Signature=zKZ6NUgLjB~mE4oDJOszxwws2U1p0UHDxb8eCIxALdYQDAnLwJL3xWoKi5GwkLW7NMTEn7ZPGOVhHq9J82nIwTF8IHY9Ib7OHDubhZHFS2RTsYcnir8C8OcEefrcqI9~2JHKCOVKqmuQXw7aQM3JAczxvwJl~Vp3KMCl7ytZ~fBZDufi1rD5dm5uFvJzVdyh7oxY6dBSvec0uvpOEkBIcMEjzo67UnRtA0G0IDQ0mh-aH8gfH~4YxOfWsTryLA8ju67qHf3FibxqWv3JvSmIKPvjWcikShTYGc3vITCrLKX0aDchqU1P5eNJ02sFxguQnoTb3atqYUgXFq9m3aAMPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
