Key Points
Mutations in the TLR/MYD88 pathway occur in 4% of patients with CLL, and they are the most frequent in young patients.
TLR/MYD88 mutations in CLL patients confer a good outcome, which is similar to that of the age- and gender-matched healthy population.
Abstract
Mutations in Toll-like receptor (TLR) and myeloid differentiation primary response 88 (MYD88) genes have been found in chronic lymphocytic leukemia (CLL) at low frequency. We analyzed the incidence, clinicobiological characteristics, and outcome of patients with TLR/MYD88 mutations in 587 CLL patients. Twenty-three patients (3.9%) had mutations, 19 in MYD88 (one with concurrent IRAK1 mutation), 2 TLR2 (one with concomitant TLR6 mutation), 1 IRAK1, and 1 TLR5. No mutations were found in IRAK2 and IRAK4. TLR/MYD88-mutated CLL overexpressed genes of the nuclear factor κB pathway. Patients with TLR/MYD88 mutations were significantly younger (83% age ≤50 years) than those with no mutations. TLR/MYD88 mutations were the most frequent in young patients. Patients with mutated TLR/MYD88 CLL had a higher frequency of mutated IGHV and low expression of CD38 and ZAP-70. Overall survival (OS) was better in TLR/MYD88-mutated than unmutated patients in the whole series (10-year OS, 100% vs 62%; P = .002), and in the subset of patients age ≤50 years (100% vs 70%; P = .02). In addition, relative OS of TLR/MYD88-mutated patients was similar to that in the age- and gender-matched population. In summary, TLR/MYD88 mutations identify a population of young CLL patients with favorable outcome.
Introduction
Chronic lymphocytic leukemia (CLL) is the most frequent leukemia in adults in Western countries and is characterized by the proliferation and progressive accumulation of mature clonal B lymphocytes in bone marrow, blood, and lymphoid tissues.1 The clinical course of the disease is highly heterogeneous, with some patients requiring early treatment of disease progression while others have an indolent course that does not affect life expectancy.2 Several biological features, including IGHV mutational status,3,4 cytogenetic abnormalities,5 or the expression of several proteins in the leukemic lymphocytes4,6,7 have been related to patient outcome. More recently, the use of next-generation sequencing (NGS) technologies has revealed new recurrent mutations in this disease that are present at relatively low (5% to 15%) (eg, NOTCH1, SF3B1, ATM) or very low frequencies (<5%) (eg, myeloid differentiation primary response 88 [MYD88], XPO1, POT1, BIRC3, SI).8-14 Despite the rarity of these new mutations, they could in part explain the CLL heterogeneity and help in identifying clinically relevant groups of patients.
MYD88 has been found recurrently mutated in different lymphoproliferative disorders,15-18 including in approximately 3% of patients with CLL.8,10 MYD88 is an adaptor protein of the interleukin-1 receptor (IL-1R)/Toll-like receptor (TLR) that has a role in innate immune response and plays a crucial role in the homeostasis of human B cells.19 After activation of TLRs, MYD88 is phosphorylated and subsequently recruits IL-1R–associated kinases (IRAKs) and other downstream proteins such as TRAF6, finally resulting in activation of the nuclear factor κB (NF-kB) pathway.8,18-20 CLL cells have a similar expression of surface TLRs and response to their activation as normal B cells, indicating that the TLR signaling framework is competent in CLL cells.21 Although other genes of this innate immune response pathway besides MYD88 have been found to be mutated in occasional cases of CLL, their integrative clinical impact has not been examined. In this study, we have investigated the presence of mutations in genes of the TLR and MYD88 (TLR/MYD88) pathway previously found to be mutated in whole-genome sequencing or whole-exome sequencing (WES) (MYD88, IRAK1, IRAK2, IRAK4, TLR2, TLR5, and TLR6),8-10 in order to define the clinicobiological characteristics and outcome of patients carrying mutations in this pathway.
Material and methods
Patients
In all, 587 patients (353 males and 234 females) diagnosed with CLL according to World Health Organization criteria1,22 with available pretreatment tumor DNA were included in the study. Main characteristics of the patients, treatment given, and outcome are detailed in supplemental Data available on the Blood Web site. Of the total number of patients, 209 (36%) died during follow-up, with a median overall survival (OS) of 12.9 years.
All the patients gave informed consent to participate in the study according to the guidelines of the International Cancer Genome Consortium CLL project and local ethics committees. This study was conducted in accordance with the Declaration of Helsinki.
Mutational analysis
Mutations of MYD88, IRAK1, IRAK2, IRAK4, TLR2, TLR5, and TLR6 were investigated by WES, as previously described,9 in 243 patients. In addition, we expanded the analysis of these genes in 344 patients by Sanger sequencing. Sanger sequencing was also performed in 130 patients in which WES was done, with full agreement in the gene status. The primers and polymerase chain reaction conditions for MYD88, IRAK1, TLR2, TLR5, and TLR6 are detailed in supplemental Table 1. Detailed information on mutational detection of NOTCH1, SF3B1, TP53, and IGHV is shown in supplemental Data.
Gene expression arrays and serum cytokines
The biological effects of mutations in TLR/MYD88 were analyzed by gene expression profile and cytokine determinations, as detailed in supplemental Data.
Statistical analysis
Fisher’s test or nonparametric tests were used to correlate clinical and biological variables according to TLR/MYD88 mutations. Time to treatment (TTT) and OS curves were plotted by the Kaplan and Meier method and compared by the log-rank test. Multivariate Cox regression analysis was used to assess the independent prognostic impact of TLR/MYD88 mutations on patient outcome.23,24 Relative survival was calculated by the cohort method described by Dickman et al.25 Estimates of expected survival were calculated by the Ederer II method26 from Spanish life tables stratified by age, sex, and calendar year, which were obtained from the Human Mortality Database (www.mortality.org). All statistical tests were two-sided, and statistical significance was considered to be significant at ≤ .05. All the analyses were conducted by using SPSS 20 (http://www-01.ibm.com/software/analytics/spss/) and Stata 11 (www.stata.com) software.
Results
Mutations in the TLR/MYD88 pathway
MYD88 mutations were observed in 19 (3.2%) of 587 patients. The most frequent mutation was L265P, which was detected in 16 (84%) of the 19 patients. In 3 patients, other MYD88 somatic mutations were found (V147L, S243N, and S219C). Additionally, 2 (0.4%) of 460 patients had IRAK1 mutations (R666* and L692F), the latter with a concurrent MYD88 L265P mutation; 2 (0.4%) of 469 patients had an identical mutation in TLR2 (D327V), and one of them had a concurrent mutation in TLR6 (P403S); 1 (0.2%) of 469 and 1 (0.2%) of 466 patients had a mutation in TLR5 (N96K). By WES, the frequency for the MYD88 mutant allele was between 40% and 60%, suggesting that the mutation was present in all tumor cells, with the exception of MYD88 V147L in which the frequency was 29%, indicating that it may be subclonal. Similarly, the allelic frequency of mutated IRAK1, TLR5, TLR6, and one of the TLR2 mutations was higher than 40%, whereas the other TLR2 mutation was subclonal (frequency, 27%). No somatic mutations in IRAK2 and IRAK4 were identified. Overall, 23 (3.9%) of 587 patients had one or more mutations in the TLR/MYD88 pathway.
Association of TLR/MYD88 mutations with initial clinicobiological features
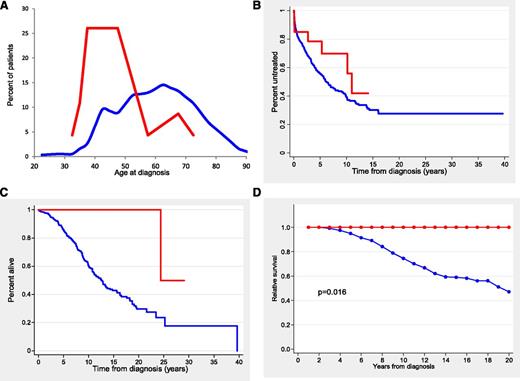
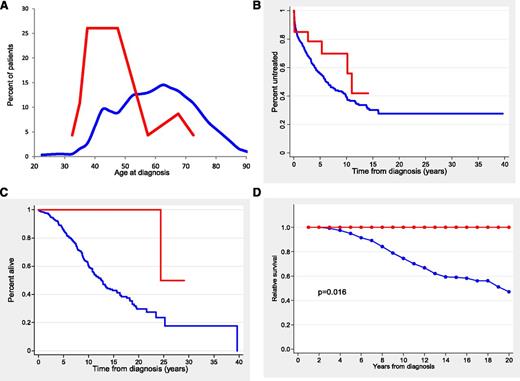
The main clinical and biological characteristics of the patients according to TLR/MYD88 mutations are listed in Table 1. Patients with TLR/MYD88 mutations were significantly younger (median age, 47 years; range, 32 to 72 years) than unmutated patients (median age, 61 years; range, 24 to 94 years) (P < .001) (Figure 1A). In fact, 19 (83%) of 23 TLR/MYD88-mutated patients were younger than age 50 years at diagnosis in comparison with 122 (22%) of 567 patients without mutations (P < .001). Patients with TLR/MYD88 mutations had more advanced Binet and Rai stages at diagnosis, more frequently mutated IGHV, low CD38 expression, low ZAP-70 expression, and a trend to higher frequency of del(13q)(q14.3) (Table 1 and supplemental Table 2). Fifty-nine of 587 patients had a small monoclonal spike in the electrophoresis assay with no differences according to TLR/MYD88. No immunoglobulin M monoclonal spike was observed in TLR/MYD88-mutated patients.
Age distribution and outcome of patients according to TLR/MYD88 mutations. (A) Histogram representing the percentage distribution of patients according to age at diagnosis for TLR/MYD88-mutated (red line) and TLR/MYD88-unmutated (blue line) CLL patients. (B) TTT in Binet stage A and B patients (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients). (C) OS (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients). (D) Relative survival (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88 unmutated patients).
Age distribution and outcome of patients according to TLR/MYD88 mutations. (A) Histogram representing the percentage distribution of patients according to age at diagnosis for TLR/MYD88-mutated (red line) and TLR/MYD88-unmutated (blue line) CLL patients. (B) TTT in Binet stage A and B patients (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients). (C) OS (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients). (D) Relative survival (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88 unmutated patients).
No differences were observed in the main clinicobiological characteristics between patients with MYD88 mutations and the 4 patients with mutations in other genes of the pathway (ie, 3 were age ≤50 years and 3 also had mutated IGHV).
Sera levels of IL-6, IL-1RA, CLL2, CLL3, CLL4, CLL5, and CLL11 did not differ according to the mutational status of TLR/MYD88. The gene expression profile of CLL with mutated TLR/MYD88 had overexpression of genes related to the NF-κB pathway (see supplemental Methods and supplemental Results).
Clinical impact of TLR/MYD88 mutational status
The outcome of the patients according to the TLR/MYD88 mutational status is described in Table 1. In patients with Binet stage A or B, the 10-year TTT of TLR/MYD88-mutated and unmutated patients was not significantly different (30% vs 62%, respectively) (Figure 1B). In addition, no differences were found in the treatment given or in the response to therapy according to TLR/MYD88 mutational status. Time to next treatment (TTNT) measured from first response was significantly longer in patients with TLR/MYD88 mutations than in those without mutations (median time, 11.5 vs 2.4 years, respectively; P = .04).
The OS of patients with TLR/MYD88 mutations was significantly longer than that of unmutated patients (10-year OS, 100% vs 62%, respectively; P = .002) (Figure 1C). Other variables associated with better OS were age ≤50 years at diagnosis, Binet stage A, low ZAP-70 expression, low CD38 expression, absence of adverse cytogenetics, mutated IGHV, absence of NOTCH1 mutation, and absence of SF3B1 mutation (P < .001 for all comparisons). A multivariate analysis that included age, gender, Binet stages, IGHV, adverse cytogenetics, SF3B1, NOTCH1, and TLR/MYD88 mutations with a final inclusion of 438 patients showed that the most important variables for predicting OS were age (relative risk [RR], 1.054; P < .0001), IGHV (RR, 4.58; P < .001), Binet stage (RR, 1.67; P < .001), and adverse cytogenetics (RR, 1.7; P = .017).
To analyze the impact of TLR/MYD88 mutations on the life expectancy of CLL patients, relative OS adjusted by general population mortality rates was calculated. As shown in Figure 1D, the life expectancy of patients with TLR/MYD88 mutations was similar to that of the age- and gender-matched population. In contrast, CLL patients with no TLR/MYD88 mutations had a decrease in life expectancy of 52% at 20 years.
Mutations in TLR/MYD88 in young patients
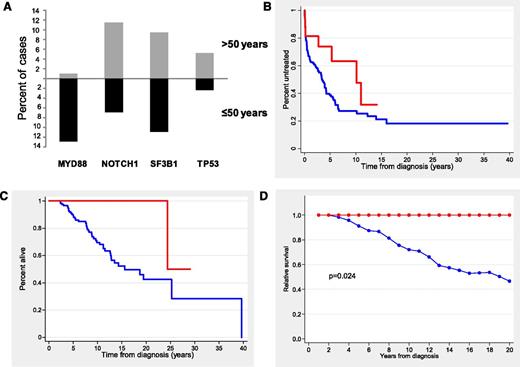
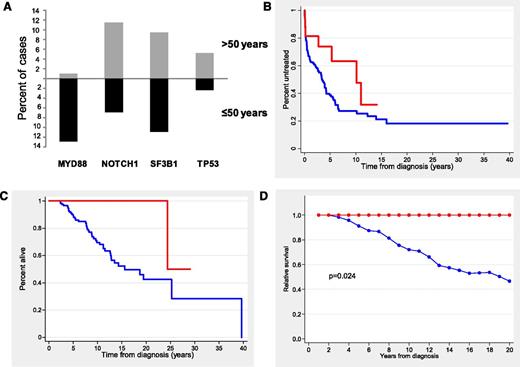
Because most patients with mutations in TLR/MYD88 were young, we restricted the analysis to the subgroup of 146 patients (25%) age ≤50 years at diagnosis. The clinical characteristics of the patients according to TLR/MYD88 mutations are shown in Table 2. Young CLL patients with TLR/MYD88 mutations had the same clinicobiological profile previously identified in the whole series with more frequent mutated IGHV, low CD38 expression, low ZAP-70 expression, and normal levels of β2-microglobulin. In addition, they showed a monoclonal spike more frequently than the remainder of the patients (21% vs 6%; P = .038). TLR/MYD88 mutations (19 [13%] of 146) were the most prevalent in young patients, followed by SF3B1 (11 [11%] of 100), NOTCH1 (9 [6.9%] of 130), and TP53 (2 [2.3%] of 86). Of note, TLR/MYD88 mutations were the only ones showing a differentiated incidence according to age (13% in patients age ≤50 years vs 0.9% in patients age >50; P < .001) (Figure 2A).
Distribution of gene mutations according to age and outcome of CLL patients age ≤50 years according to TLR/MYD88. (A) Distribution of the main gene mutations according to age at diagnosis (≤50 vs >50 years). (B) TTT in Binet stage A and B patients (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients). (C) OS (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients). (D) Relative survival (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients).
Distribution of gene mutations according to age and outcome of CLL patients age ≤50 years according to TLR/MYD88. (A) Distribution of the main gene mutations according to age at diagnosis (≤50 vs >50 years). (B) TTT in Binet stage A and B patients (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients). (C) OS (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients). (D) Relative survival (red line, TLR/MYD88-mutated patients; blue line, TLR/MYD88-unmutated patients).
One hundred two patients (70%) eventually required treatment, with no significant differences observed according to TLR/MYD88 mutations in Binet stages A and B (10-year TTT, 37% vs 74% for mutated and unmutated patients, respectively; P = .07) (Figure 2B). Response to treatment was not significantly different according to TLR/MYD88. TTNT was significantly longer in patients with TLR/MYD88 mutations than in those without mutations (median time, 11.5 vs 2.0 years, respectively; P = .02).
After a median follow-up of 9.2 years (range, 0.1 to 26 years), 45 patients had died with a 10-year OS of 73%. OS was significantly better for patients with TLR/MYD88 mutations than for the remainder of the patients (10-year OS, 100% vs 70%, respectively; P = .02) (Figure 2C). Other variables predicting better OS were Binet stage A, normal lactate dehydrogenase, normal β2-microglobulin, mutated IGHV, low CD38 expression, low ZAP-70 expression, absence of NOTCH1 mutations, and absence of SF3B1 (P < .05 for all comparisons). In a multivariate analysis including gender, age, Binet stages, adverse cytogenetics, IGHV, and TLR/MYD88, only the mutational status of IGHV independently predicted OS (RR, 9.3; P < .001).
Finally, in the group of young patients, the calculation for relative OS was adjusted by age- and gender-matched population mortality rates. As depicted in Figure 2D, the life expectancy of patients with TLR/MYD88 mutations was similar to that of the general population. In contrast, CLL patients with no TLR/MYD88 mutations had a decrease in life expectancy of 54% at 20 years.
Discussion
CLL is a heterogeneous disease with subsets of patients that have different clinical behaviors. The main predictors of CLL aggressiveness currently recognized are cytogenetic abnormalities and the IGHV mutational status.27 The recent identification of a set of new recurrent somatic mutations using NGS8-12 has expanded the possibilities to further delineate the clinicobiological characteristics of CLL, define new predictors, and identify new therapeutic targets. The most frequent recurrent mutations in CLL patients are NOTCH1 and SB3F1, detected in approximately 5% to 15% of patients at diagnosis28,29 and associated with poor outcome.10,30-32 In this scenario, the inclusion of some of these mutations in the stratification of risk of patients with CLL is warranted, and a model that integrates these mutations has been recently proposed.33
NGS studies have revealed mutations in MYD88 (3% to 5%) along with other genes of the same pathway at lower frequencies.8-12 TLRs trigger an innate immune response in B cells after recognition of different pathogen-associated molecular patterns. Activation of this pathway is also considered a bridge between the innate and adaptive immunologic responses by providing costimulatory signals for the BCR.34,35 CLL cells express a similar pattern of TLRs as normal memory B cells, and their stimulation triggers the activation of the NF-κB and MAPK signaling pathways protecting CLL cells from spontaneous apoptosis.34-36 The activity of TLRs is negatively regulated by the type I transmembrane inhibitory receptor TIR8. In the TCL1 murine model of CLL, transgenic mice lacking TIR8 show an accelerated appearance of monoclonal B-cell expansions and a shorter survival experimentally supporting the implications of TLR activation in the pathogenesis of CLL.37 Thus, all of the previous observations highlight the relevance of the TLR pathway in the development and progression of CLL, and this idea is reinforced by the finding of recurrent somatic mutations in genes from this pathway by NGS analysis. Concordantly, in this study, we have observed that CLL with mutations in TLR/MYD88 had a significant overexpression of genes of the NF-κB pathway, and that these patients have an early appearance of CLL.
MYD88 is a critical adaptor protein of the IL-1R/TLR signaling pathway.19 The MYD88 mutations described here have also been observed in other series of CLL and diffuse large B-cell lymphoma studies,8,10,18,33,38 and a gain of function has been demonstrated for most of them (ie, MYD88 L265P, MYD88 S243N, MYD88 S219C, and TLR2 D327V) triggering an increased production of chemokines.8,18,39 The remaining mutations appeared in highly conserved residues, and PolyPhen score predicted a functional damage impact in all cases. Lymph node microenvironment and cytokines play a crucial role in CLL proliferation.40-42 However, in spite of the in vitro cytokine overproduction of the mutated cells, sera levels were similar among TLR/MYD88-mutated and -unmutated patients, suggesting a local effect that would be consistent with the higher frequency of Rai stages I and II in mutated patients.
TLR/MYD88 mutations were the most frequent in CLL patients age ≤50 years reaching an incidence of 13%. It is notable that these are the only mutations described in CLL with differentiated incidence according to age. In contrast to patients with NOTCH1 or SF3B1 mutations, patients with TLR/MYD88 mutations had favorable biological characteristics and outcomes. Although no differences were observed in terms of TTT, TTNT was considerably longer in these patients. Regarding survival, patients with mutated TLR/MYD88 CLL showed remarkably good OS. Such a favorable prognosis is not merely due to age, because it was also observed in patients age ≤50 years. Keeping in mind the low number of patients with mutations, it is not surprising that the impact on OS was not independent of other well-known prognostic factors.
A recent study of the characteristics of young CLL patients (age ≤55 years)43 showed a higher frequency of adverse clinicobiological features and a shorter OS when compared with a sex- and age-matched population. However, the authors identified a small subgroup (15%) of young CLL patients (Rai stage 0, mutated IGHV, and favorable fluorescence in situ hybridization results) with an OS similar to that of the general population. Interestingly, young patients with TLR/MYD88 mutations, even if they are at a more advanced clinical stage, had a relative OS indistinct from that of the general population, whereas a reduction of half in life expectancy was observed in young CLL patients without mutations in this pathway. This emphasizes the clinical relevance of these mutations, which can identify approximately 10% to 15% of young CLL patients who have a very favorable outcome.
In summary, the analysis of recurrent mutations first identified by NGS could help to recognize subgroups of patients with particular features and outcomes. The current data highly suggest that TLR/MYD88 mutations are able to distinguish a subgroup of patients with very favorable prognosis among young CLL patients. Because of the low incidence of these mutations, large series of patients, most likely in collaborative multicenter studies, are necessary to reach enough statistical power to determine its real impact in the management of patients with CLL.
Presented in part at the 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 19-22, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded by the Spanish Ministry of Economy and Competitiveness through the Instituto de Salud Carlos III (ISCIII) (International Cancer Genome Consortium-Chronic Lymphocytic Leukemia Genome Project), Red Temática de Investigación del Cáncer of the ISCIII (RD12/0036/0036 [E. Campo], RD12/0036/0023 [A.L.-G.], RD12/0036/0004 [D.C.], RD12/0036/0069 [M.G.-D.], PIE13/00033 [E. Campo and A.L.-G.], and RD12/0036/0067 [C.L.-O.]), Plan Nacional (SAF12/38432), and Generalitat de Catalunya (2009-SGR-992). E. Campo is a researcher of the Academia Program of the Institució Catalana de Recerca i Estudis Avançats of the Generalitat de Catalunya. C.L.-O. is an investigator of the Botín Foundation.
Authorship
Contribution: M.P., A.N., V.Q., D.C., X.S.P., and C.L.-O. performed sequencing analysis; M.R., N.V., M.G.-D., J.M.H.-R., B.N., C. Rayón, E. Colado, and E. Campo reviewed the pathologic data and confirmed the diagnosis; M.P. and P.J. performed the microarray analysis; M.J. performed the serum cytokine analysis; A.M.-T., J.D., E.G., A.R.P., M.J.T., and A.L.-G. reviewed clinical data; M.A. prepared and supervised the bioethics requirements; C. Rozman contributed to the statistical analysis; E. Campo, N.V., and A.L.-G. directed the research; N.V., E. Campo, and A.L.-G. wrote the manuscript; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Neus Villamor, Unitat d’Hematopatologia, Servei d’Anatomia Patològica, Hospital Clínic de Barcelona, Institut d’Investigacions Biomèdiques August Pi i Sunyer, 08036 Barcelona, Spain; e-mail: villamor@clinic.cat.
References
Author notes
A.M.-T. and M.P. contributed equally to this study.