In this issue of Blood, Nogami et al report on a novel factor V (FV) gene mutation (FV Trp1920→Arg, FVNara) associated with activated protein C (APC) resistance and a severe thrombotic phenotype in a young Japanese patient.1 Since the affected amino acid residue is located in the light chain of FV, far from the known APC-cleavage sites, this discovery may afford new insights into the molecular mechanisms of APC resistance.
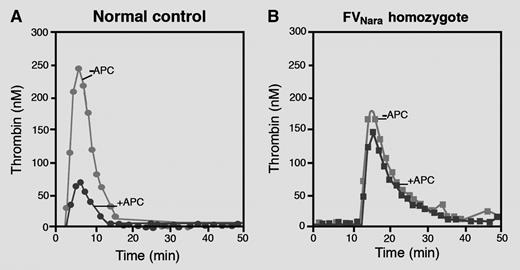
Thrombin generation curves obtained in platelet-poor plasma from a normal control (A) and from the FVNara homozygous patient (B) in the absence and presence of APC. Plasma from the FVNara homozygous patient is completely insensitive to the anticoagulant action of APC. Professional illustration by Marie Dauenheimer.
Thrombin generation curves obtained in platelet-poor plasma from a normal control (A) and from the FVNara homozygous patient (B) in the absence and presence of APC. Plasma from the FVNara homozygous patient is completely insensitive to the anticoagulant action of APC. Professional illustration by Marie Dauenheimer.
The serine-protease APC plays a major anticoagulant role by proteolytically inactivating factors Va (FVa) and VIIIa (FVIIIa), the essential cofactors of the prothrombinase and intrinsic tenase complexes, respectively. Both reactions are greatly stimulated by anionic phospholipids and by the APC-cofactor protein S. APC cleaves FVa at Arg306, Arg506, and Arg679, all located in the heavy chain of the protein, whereas the light-chain anchors FVa to the membrane surface. On the other hand, APC also cleaves the inactive precursor FV (particularly at Arg506), converting it into a still poorly defined anticoagulant cofactor that stimulates the inactivation of FVIIIa by the APC/protein S complex. Therefore, FV(a) is both a substrate and a cofactor of APC (reviewed in Castoldi and Rosing2 ).
In 1993, a reduced anticoagulant response of plasma to APC (APC resistance) was first described in a thrombophilic family3 and quickly recognized as the most common risk factor for venous thrombosis in the Caucasian population. Soon afterward, the FV Arg506→Gln (FVLeiden) mutation was identified as the underlying genetic defect.4 This mutation eliminates the APC-cleavage site at Arg506, thereby hampering not only the APC-mediated inactivation of FVaLeiden (which relies entirely on the protein S–dependent cleavage at Arg306), but also the conversion of FVLeiden into an APC cofactor for FVIIIa inactivation. The FVLeiden mutation is present in ∼5% of Caucasians, but is virtually absent in the indigenous populations of Africa, America, Eastern Asia, and Australia. More recently, other FV gene mutations associated with APC resistance have been identified in various populations, including Arg306→Thr (FVCambridge), Arg306→Gly (FVHong Kong), Ile359→Thr (FVLiverpool), and Glu666→Asp (reviewed in Castoldi and Rosing2 ). Remarkably, all of these variants predict amino acid changes at or close to the APC-cleavage sites in the heavy chain of FV(a), and their mechanisms of action can be rationalized in terms of reduced cleavage at these sites.
The interesting study by Nogami et al describes a 13-year-old Japanese boy who developed recurrent venous thrombosis during oral anticoagulant treatment.1 The patient had reduced FV levels (40 IU/dL antigen, 10 IU/dL activity) and pronounced APC resistance (see figure), prompting FV gene sequencing. This revealed a novel homozygous missense mutation (Trp1920→Arg, FVNara) in the C1 domain of FV, which was not found in 50 healthy Japanese people. In line with the observations made in the patient, recombinant FVNara showed reduced expression in conditioned media (∼50% of wild-type FV) and conferred APC resistance to reconstituted FV-deficient plasma. Moreover, detailed characterization of the mutant in model systems indicated that: (1) the APC-mediated inactivation of FVaNara is severely impaired and hardly sensitive to stimulation by protein S; and (2) FVNara expresses no APC-cofactor activity in FVIIIa inactivation. In fact, in the presence of protein S, recombinant FV(a)Nara was inactivated more slowly and expressed less APC-cofactor activity than recombinant FV(a)Leiden, convincingly proving that FVNara confers APC resistance.
Because the FVNara mutation is located in the light chain of FV(a), far from the known APC-cleavage sites, the molecular mechanism by which it causes APC resistance is unclear. However, the extensive functional data presented by Nogami et al do allow some speculations. First of all, Trp1920 is located in the hydrophobic core of the C1 domain of FV and is absolutely conserved from fish to mammals, pointing at an important structural role. Replacement of this Trp by an Arg might alter the domain structure and interfere with the secretion and/or stability of FV, as suggested by the reduced expression of FVNara observed both in vivo and in vitro. Consequently, the mutation could also modify the functional properties of FV(a). For example, since the C1 domain of FV is known to contain a membrane-binding site,5 FVNara could interfere with the binding of FV(a) to phospholipids, either directly or by altering the C1 domain structure. This would account for the resistance of FVaNara to APC-mediated inactivation, its insensitivity to protein S stimulation, and its impaired APC-cofactor activity. However, the authors present suggestive evidence, based on an enzyme-linked immunosorbent assay and a prothrombinase-based assay, that FVNara has a normal phospholipid-binding capacity. While this awaits confirmation by direct binding assays (eg, surface plasmon resonance), it is interesting to note that the common FV Asp2194→Gly polymorphism (tagging the FV HR2 haplotype), which decreases the FV level and shifts the ratio between the two C2 domain glycosylation isoforms in favor of the isoform with lower affinity for phospholipids (FV1), has been associated with mild APC resistance.6 Moreover, low FV levels and reduced phospholipid-binding capacity of FV have been recently shown to be relatively common and to be associated with a high risk of deep vein thrombosis (odds ratio >6) in the Japanese population.7
Alternatively, as proposed by Nogami et al, FVNara could cause APC resistance through defective interaction with APC and/or protein S. Although little is known on the binding sites of APC and protein S on FV(a), this is an exciting hypothesis which definitely deserves follow-up. If binding assays showed that FVNara indeed has reduced affinity for APC and/or protein S, this would not only account for the associated APC resistance, but also help to map these elusive interaction sites. Moreover, it may finally shed some light on the structural requirements for the expression of the APC-cofactor activity of FV, which is completely abolished in FVNara and whose molecular bases are still largely unknown.
These new insights will greatly enhance our understanding of the role(s) of FV(a) in the protein C pathway, as the discoveries of APC resistance and FVLeiden did 20 years ago.
Conflict-of-interest disclosure: The author declares no competing financial interests.