Key Points
The quality of evidence pertaining to the risk of recurrent thrombosis among patients with an antiphospholipid antibody is very low.
Additional studies are needed to define the impact of APLA testing on clinical decision-making.
Abstract
Laboratory evidence of antiphospholipid antibodies (APLA) in patients with a first episode of venous thromboembolism (VTE) is often considered an indication for indefinite anticoagulant therapy, but it is uncertain if this practice is justified. We performed a systematic review to determine whether the presence of APLA in patients with a first VTE is associated with an increased risk of recurrence. We searched PubMed, CINAHL, Cochrane, EMBASE, and Web of Knowledge through February 2012 and included prospective studies that met prespecified design criteria. There were 109 recurrent VTE in 588 patients with APLA and 374 recurrent VTE in 1914 patients without APLA (relative risk 1.41; 95% confidence interval [CI], 0.99 to 2.36). The unadjusted risk ratio for recurrent VTE after stopping anticoagulant therapy in patients with an anticardiolipin antibody was 1.53 (95% CI, 0.76-3.11), and with a lupus anticoagulant was 2.83 (95% CI, 0.83-9.64). All studies had important methodologic limitations and we judged the overall quality of the evidence as very low. Although a positive APLA test appears to predict an increased risk of recurrence in patients with a first VTE, the strength of this association is uncertain because the available evidence is of very low quality.
Introduction
Antiphospholipid antibodies (APLA) bind to cardiolipin or to phospholipid-associated plasma proteins such as β 2-glycoprotein I, and prothrombin or annexin A5.1 APLA occur in a variety of medical conditions (including syphilis and systemic lupus erythematosus) as well as in persons without illness.2,3 Whether detected as a lupus anticoagulant (LA), an anticardiolipin antibody (ACLA), or as an anti-beta2 glycoprotein 1 antibody, APLA have been associated with arterial and venous thrombosis4,5 and with pregnancy loss.6 Patients are defined as having the “antiphospholipid syndrome” (APS) if they have had 1 or more thrombotic events and have a positive APLA test on more than 1 occasion at least 12 weeks apart.7 APS can also be defined by certain obstetrical events.
Depending on how a positive test is defined, APLA can be found in up to 25% of patients with venous thromboembolism (VTE),8,9 and it is often recommended that these patients receive indefinite anticoagulant therapy.10-12 This recommendation is based on reports of a high risk of recurrent thrombosis in patients with APLA, particularly after stopping anticoagulant therapy. However, many of these reports describe the experience of clinics that specialize in the management of difficult cases of thrombosis; consequently, selection bias may overestimate associations between a positive APLA test and recurrent VTE. To address uncertainty surrounding the role of APLA testing in patients who have experienced VTE, we performed a systematic review to determine if the presence of APLA is associated with an increased risk of recurrence in patients with a first VTE.
Methods
Eligibility criteria
We included prospective cohort studies and randomized studies analyzed as prospective cohorts to evaluate the association of interest. Included studies should have satisfied the following criteria: (1) enrolled patients before starting follow-up (this had to have been explicitly stated, or patients had to have provided written consent before starting follow-up); (2) ≥80% of patients had a deep vein thrombosis (DVT) of the legs or a pulmonary embolism (PE); (3) ≥80% of VTE events were a first episode; (4) all patients were tested for APLA (ie, LA, ACLA, or both) on at least 1 occasion; (5) APLA testing was done on blood samples that were obtained before recurrent episodes of VTE; (6) all patients (ie, those with and without APLA) were followed to detect recurrent VTE; and (7) neither knowledge of recurrent VTE during follow-up nor knowledge of APLA status could have influenced enrollment. We were prepared to include studies published only as abstracts if the abstract reported the methods and results with sufficient detail to allow evaluation of study eligibility.
Search
We searched PubMed, CINAHL, Cochrane, EMBASE, and Web of Knowledge up until February 24, 2012, and updated the searches January 28, 2013. In PubMed, the MeSH terms “antiphospholipid syndrome,” “antibodies, antiphospholipid,” and “antibodies, anticardiolipin,” plus keywords for those concepts and for Hughes syndrome, anti b2 glycoprotein, antiprothrombin antibodies, and LA, were ANDed with MeSH terms for “venous thromboembolism,” “venous thrombosis,” and “ pulmonary embolism” plus keywords for those concepts. In addition, we ANDed those searches with the MeSH term “recurrence” plus keywords for recurrence and risk of recurrence. We used the corresponding thesaurus terms and/or keywords in CINAHL, EMBASE, Cochrane, and Web of Knowledge. We applied no language, publication date, publication type, patient age, or gender limits.
We also searched the reference lists of included studies and our personal files. We attempted to contact experts in the field and asked them to verify the completeness of our list of included studies based on our predefined eligibility criteria. We searched the Web of Knowledge for publications that cited any of our included studies (forward searching). Finally, we searched the “gray literature” (specific strategy available on request). We registered the protocol for this review (#CRD42011001817) at the Prospero international register of systemic reviews (http://www.crd.york.ac.uk/Prospero/) on December 12, 2011.
Selection
Two reviewers screened in duplicate and independently the titles and abstracts for eligibility. We retrieved the full text of all citations that at least 1 reviewer judged as potentially eligible. The 2 reviewers then screened in duplicate and independently the full text articles for eligibility and resolved disagreements by discussion or with the help of a third reviewer.
Data extraction
Two reviewers independently extracted data from every included study. After entering the data, they exchanged the completed tables, identified discrepant data, and double-checked those data. If there were still discrepancies between the 2 authors, these were resolved through discussion. For studies with incompletely reported data, we attempted to contact the authors.
We extracted data on study design, demographics of enrolled patients, whether patients were followed while on or after stopping anticoagulant therapy and for how long, losses during follow-up, when APLA testing was performed, which types of APLA were tested for and using what method, presence of factors with the potential to confound the association between APLA and outcomes during follow-up, and the number of patients with each type of APLA and with each study outcome (Tables 1 and 2).
Our primary outcome of interest was the relative risk (RR) of recurrent VTE after anticoagulant therapy was stopped in patients with compared with patients without APLA. If assessed and reported, we also planned to extract data on: recurrent VTE while on anticoagulant therapy; arterial thrombosis; the proportion of time that the International Normalized Ratio was in the study’s target range during anticoagulant therapy; bleeding during anticoagulant therapy; development of the postthrombotic syndrome; and death. We also prespecified subgroup analyses to look for an association between either ACLA or LA and recurrent VTE.
We used the following individual study characteristics to judge the potential risk of bias: (1) whether APLA positivity was predefined; (2) the possibility that patients were tested for APLA before potential enrollment and were not enrolled because APLA were found (ie, may have remained on indefinite anticoagulant therapy); (3) the lack of independent outcome adjudication; (4) the lack of blinding to APLA status of patients, health care providers, outcome assessors, and outcome adjudicators; and (5) timing of the blood draw for APLA testing (Table 2). Finally, a global assessment for the risk of bias (low, moderate, or high) was generated using the grid described in the Quality in Prognosis Studies (QUIPS) tool (Table 2).13
Data synthesis
We planned to analyze adjusted effect estimates. However, because only 1 study14 described an adjustment for possible confounding variables, we had to meta-analyze unadjusted effect estimates (risk ratios [RRs]). We used the number of outcome events and the number of patients assessed for that outcome in each study arm (APLA positive and APLA negative) to calculate the RRs for each study. The primary analysis was a comparison of patients with any APLA, as defined by each study, to patients with no APLA. We also prespecified that we would analyze separately, where data are available, patients with and without an ACLA, and patients with and without an LA.
We then pooled the RRs from individual studies using a random-effects model (Review Manager Version 5.1). We evaluated heterogeneity across trials using the I2 statistics, which describes the percentage of total variation across studies that is not due to chance, and by calculating the P value for heterogeneity from the χ-squared test. Finally, we examined the association between any APLA and recurrent VTE in studies that enrolled only patients with an unprovoked first VTE.
Results
Literature search and study selection
The initial search in January 2012 identified 2074 unique citations; the search update in January 2013 retrieved 134 additional unique citations. Title and abstract screening of all citations identified 60 potentially eligible studies that both (n = 14) or 1 (n = 44) of the 2 reviewers considered potentially eligible. Full screening of the full texts of the 60 studies identified 8 as eligible (Figure 1).8,14-20 We identified no additional eligible studies by: (1) searching the reference lists of included studies and our personal files; (2) contacting 10 experts (see Acknowledgments); (3) searching Web of Knowledge for publications that cited any of the 8 eligible studies forward searching; and 4) searching multiple gray literature sources using the keywords enumerated previously. The search update in January 2013 retrieved 134 additional unique citations; none of these was eligible.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Study characteristics and methodological quality
The 8 studies included 6 randomized trials and 2 cohort studies and a total of 3114 patients (Tables 1 and 2). The randomized studies were analyzed as prospective cohorts to evaluate the association of interest (as opposed to analysis by randomized arms to assess the effectiveness of an intervention). One of the randomized studies14 was a pooled analysis of 2 previously reported randomized trials21,22 ; we report this pooled analysis as a single study.
Testing for APLA varied with respect to the antibody sought (ACLA [immunoglobulins G, M, or A] and/or LA; some studies tested for only 1 type of APLA, whereas others tested for both types (Table 1). For analyses that focused on a specific type of APLA (eg, ACLA, LA), the comparator group had no APLA in some studies (ie, other APLA tested for and excluded if found), whereas in other studies, the comparator group could include patients with another type of APLA (ie, not tested for or not excluded if tested for and found).
Testing for an APLA also varied with respect to: (1) the assay used to detect each type of antibody; (2) the criteria used to define a positive test; 3) whether APLA had to be confirmed on a second occasion; (4) whether blood was obtained when patients were on or off anticoagulant therapy; and (5) the interval between diagnosis of the original VTE and the collection of blood for testing (Table 1). APLA testing was performed in central laboratories in all but 1 study.14 Symptomatic recurrent VTE in APLA-positive and APLA-negative patients was reported by all studies; however, no study reported the risk of bleeding, death, or post-thrombotic syndrome according to APLA status.
Follow-up occurred (1) after anticoagulants were stopped in 3 studies14,16,18 and in 1 of 2 randomized groups in a fourth study19 ; (2) mostly after anticoagulants were stopped with some follow-up before anticoagulants were stopped (separate data were not reported for when patients were and were not anticoagulated) in 3 studies15,17,20 ; (3) during anticoagulation in 1 of 2 randomized groups in 1 study19 ; and (4) during and after anticoagulation of the same patients, with outcomes reported separately for each period in 1 study.8
Three studies included only patients with unprovoked VTE,14,16,18 whereas 5 studies included both patients with provoked as well as unprovoked VTE.8,15,17,19,20 Two studies described the proportion of patients with cancer; they included 3% and 5% of patients with malignancy, respectively15,19 (Table 3). The original episode of VTE was either a DVT (usually proximal) or a PE in 7 studies,8,14,16-20 and had to be a DVT in 1 study.15 Patients who were known to have APLA (there was no routine preenrollment screening in any study) were excluded from enrollment in 4 studies,14,16-18 and not excluded in 2 studies8,15 ; this information was not available for 2 studies.19,20
All but 1 study used central adjudication of outcomes.8 Blinding of patients, health care providers, study outcome assessors, and central adjudicators to APLA status was required in 6 studies and uncertain in 2.14,15 Losses to follow-up were not reported in 5 studies.8,14-16,19 In the remaining 3 studies, fewer than 5% of patients were lost to follow-up (Table 2).17,18,20 Only 1 study described adjustment for confounding. However, that published manuscript does not include an adjusted odds ratio; it states that the adjusted results were not different from the unadjusted analysis.14
Table 2 presents the results of global assessment for the risk of bias using the QUIPS tool.13 Of the 8 studies, 1 was judged to have a low risk of bias, 5 were judged to have a moderate risk of bias, and 2 were judged to have a high risk of bias. There were inadequate data to assess APLA as a risk factor for bleeding, development of the postthrombotic syndrome, or mortality.
Recurrent VTE with APLA vs No APLA
Patients not on anticoagulants.
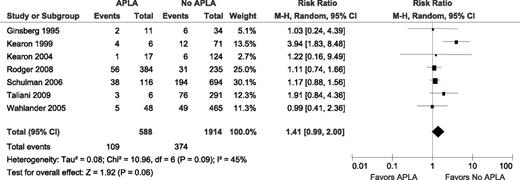
The 8 studies reported on the association between an APLA (ACLA, LA, or both) and recurrent VTE in patients who were not on anticoagulants (Table 3). The study by Bank et al, which included the smallest number of patients, reported no episodes of VTE in 7 patients with APLA. Therefore, a RR could not be calculated for this study and it was not included in the meta-analysis (Figure 2). In the 7 included studies, there were 109 recurrent VTE in 588 patients with APLA and 374 recurrent VTE in 1914 patients without APLA, yielding an unadjusted RR of 1.41 (95% confidence interval [CI] 0.99-2.36; I2 = 49%; P for heterogeneity = .09).
Relative risks for recurrent VTE after stopping anticoagulant therapy with APLA vs without APLA. M-H, Mantel–Haenszel.
Relative risks for recurrent VTE after stopping anticoagulant therapy with APLA vs without APLA. M-H, Mantel–Haenszel.
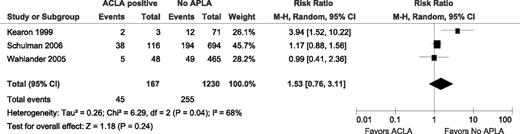
Six studies specified whether or not patients had ACLA.8,15-17,19,20 In 3 of these, however, there were no episodes of VTE in a total of 27 patients with ACLA (Table 3). Therefore, a RR could not be calculated for these 3 studies and they are not included in the meta-analysis for this subgroup (Figure 3). In the 3 included studies, there were 45 recurrent VTE events in 169 patients with ACLA and 255 recurrent VTE in 1230 patients without APLA, yielding an unadjusted RR of 1.53 (95% CI 0.76-3.11; I2 = 68%; P for heterogeneity = .04).
Relative risks for recurrent VTE after stopping anticoagulant therapy with ACLA vs without APLA. M-H, Mantel–Haenszel.
Relative risks for recurrent VTE after stopping anticoagulant therapy with ACLA vs without APLA. M-H, Mantel–Haenszel.
Four studies specified whether or not patients had an LA.8,15-17 In 1 of these, however, there were no episodes of VTE in 2 patients with an LA(Table 3). Therefore, a RR could not be calculated for this study and it is not included in the meta-analysis (Figure 4). In the 3 included studies, there were 5 recurrent VTE in 19 patients with an LA and 24 recurrent VTE in 229 patients without an APLA, yielding an unadjusted RR of 2.83 (95% CI 0.83-9.64; I2 = 0%; P for heterogeneity = .44).
Relative risks for recurrent VTE after stopping anticoagulant therapy with an LA vs without APLA. M-H, Mantel–Haenszel.
Relative risks for recurrent VTE after stopping anticoagulant therapy with an LA vs without APLA. M-H, Mantel–Haenszel.
Three of the studies included only patients with an unprovoked VTE (Table 3).14,16,18 In these 3 studies, there were 63 recurrent VTE in 396 patients with APLA and 119 recurrent VTE in 597 patients without APLA, yielding an unadjusted RR of 1.94 (95% CI 0.84-4.46; I2 = 79%; P for heterogeneity = .008) for this subgroup of patients.
Patients on anticoagulants.
Two studies reported on the association between APLA (ACLA, LA, or both) and recurrent VTE in patients who were on anticoagulants.8,19 The study by Ginsberg reported no episodes of VTE in 16 patients with APLA. Therefore, a RR could not be calculated for this study. In the study by Walhander, among patients randomized to extended anticoagulation with ximelagatran, there were 2 recurrent VTE in 45 patients with APLA (only ACLA was assessed) and 5 recurrent VTE in 483 patients without an ACLA, yielding an unadjusted RR of 3.16 (95% CI 0.75-13.3).
Discussion
This meta-analysis of 8 studies found evidence suggesting that patients with a first VTE who have APLA have a higher risk for recurrent VTE when compared with patients without APLA. However, because the evidence is of very low quality, the findings of our analysis are not definitive. The risk of recurrence after stopping anticoagulant therapy appears to be about 40% higher with APLA, but the 95% CI around this estimate includes both no increase as well as a more than 2-fold increase in VTE risk.
The strengths of our study include pre-defined clear eligibility criteria and a systematic approach to study selection, data abstraction, and risk of bias assessment. All studies suffered from the risk of bias (Table 2) related to a number of factors such as the lack of appropriate handling of confounding. Also, there was no consistency in how APLA testing was performed: blood samples for testing were obtained at different times relative to when the index case of VTE was diagnosed; some samples were obtained while patients were still anticoagulated; testing was for different types and combinations of APLA; and when the same or similar tests were performed, criteria differed on when to categorize results as positive. Differences in the APLA tests that were performed, and the criteria that were used to categorize test results as abnormal probably explain the inter-study variability in proportion of patients with an APLA. In the study by Rodger and colleagues, for example, 62% of the patients were counted as having APLA because a very low anticardiolipin cutoff was used. The difference in APLA measurement among the studies in this analysis highlights the variability with which APLA positivity may be defined, both in clinical practice and in research settings. For example, none of the included studies reported on the use of anti-beta2 glycoprotein 1 antibodies, a test that is now commonly performed when APS is suspected.
Several characteristics of the included studies may have led us to underestimate the strength of the association between APLA and VTE recurrence. First, some of the included studies excluded patients with known APLA, Second, patients with more severe presentations of VTE may have been more likely to be tested for APLA. Third, the definition of APLA positivity was problematic: none of the studies required repeat testing, as recommended by the International Society on Thrombosis and Haemostasis,7 and some used nonstandard cutoff values. We also acknowledge that much of the experience in this area initially came from retrospective studies of patients who had thrombosis in association with collagen-vascular disease, and the studies cited in this meta-analysis likely include relatively few such patients. Furthermore, we were unable to assess if repeatedly positive APLA testing was more strongly associated with recurrent VTE than a single positive test or negative testing because none of the studies required that APLA be positive on more than 1 occasion. Diagnostic criteria for the APS, established by the International Society on Thrombosis and Haemostasis, require that laboratory testing be positive on at least 2 occasions at 12 weeks apart.7
There is evidence that an LA is more strongly associated with thrombosis than a positive ACLA test.23 Our analysis was consistent with a stronger association between an LA and recurrent VTE than for ACLA (Figures 3 and 4). A recent study suggests that being positive for an LA, ACLA, and an anti-beta2 glycoprotein 1 antibody (ie, “triple positive”) confers a higher risk of thrombosis than having only 1 persistently abnormal APLA test.5 This observational study was not eligible for our analysis because patients were not enrolled prospectively.
Patients with first VTE provoked by a reversible risk factor, and those with an unprovoked isolated distal DVT, have a low risk of recurrence and are generally anticoagulated for 3 months.24 Patients with a first unprovoked proximal DVT or PE have a much higher risk of recurrence and may benefit from extended anticoagulant therapy, depending on both the risk of bleeding as well as the patient’s individual preferences.24 Because our findings were not definitive, this analysis is unable to determine if decisions about the duration of anticoagulation in patients with VTE (whether provoked by a reversible risk factor or unprovoked) should be altered by the results of APLA testing.
We propose that future studies of the natural history of VTE, rather than excluding patients with APLA, should assess the influence of APLA on the risk of recurrence. These studies should also assess the risk of recurrence with different subtypes of APLA and determine if recurrence risk varies according to whether APLA testing was transiently or persistently positive. However, until the results of more methodologically rigorous studies are available, the role of APLA testing in patients with a first VTE will remain uncertain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the following experts who responded to our inquiry about additional studies that might meet our eligibility criteria but were not identified by our original search: Giancarlo Agnelli, Mark Crowther, Philip G. de Groot, Sabine Eichinger, Jeffrey Ginsberg, Saskia Middeldorp, Thomas Ortel, Vittorio Pengo, Frits Rosendaal, and Paul Ridker.
This project was completed without funding and ethics committee approval was not necessary because this study pooled data already available in the public domain.
Authorship
Contribution: D.G., E.A.A., R.C., and C.K. participated in the design of the systematic review; R.C. performed the literature search; D.G. and C.K. screened titles and abstracts of available studies and abstracted the data from those that were eligible for inclusion; E.A.A. pooled the data and performed other statistical analysis; and all authors made significant contributions to the writing of the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Garcia, 1100 Fairview Ave N, D5-100, PO Box 19024, Seattle, WA 98109; e-mail: davidg99@u.washington.edu.