Key Points
Epo-induced EpoR internalization is mediated through a novel Cbl/p85/epsin-1 pathway.
Mutated EpoR in primary familial and congenital polycythemia patients cannot activate this pathway, exhibiting excessive Epo signaling.
Abstract
Erythropoietin (Epo) binding to the Epo receptor (EpoR) elicits downstream signaling that is essential for red blood cell production. One important negative regulatory mechanism to terminate Epo signaling is Epo-induced EpoR endocytosis and degradation. Defects in this mechanism play a key role in the overproduction of erythrocytes in primary familial and congenital polycythemia (PFCP). Here we have identified a novel mechanism mediating Epo-dependent EpoR internalization. Epo induces Cbl-dependent ubiquitination of the p85 regulatory subunit of PI3K, which binds to phosphotyrosines on EpoR. Ubiquitination allows p85 to interact with the endocytic protein epsin-1, thereby driving EpoR endocytosis. Knockdown of Cbl, expression of its dominant negative forms, or expression of an epsin-1 mutant devoid of ubiquitin-interacting motifs all compromise Epo-induced EpoR internalization. Mutated EpoRs mimicking those from PFCP patients cannot bind p85, co-localize with epsin-1, or internalize on Epo stimulation and exhibit Epo hypersensitivity. Similarly, knockdown of Cbl also causes Epo hypersensitivity in primary erythroid progenitors. Restoring p85 binding to PFCP receptors rescues Epo-induced epsin-1 co-localization and EpoR internalization and normalizes Epo hypersensitivity. Our results uncover a novel Cbl/p85/epsin-1 pathway in EpoR endocytosis and show that defects in this pathway contribute to excessive Epo signaling and erythroid hyperproliferation in PFCP.
Introduction
In normal adults, ∼200 billion erythrocytes are produced daily to replace senescent erythrocytes, ensuring adequate tissue oxygenation. Under stress conditions such as being at a high altitude or in response to anemia or hemorrhage, the erythropoiesis rate can increase up to 10-fold. Erythropoietin (Epo) is the primary cytokine that drives red blood cell production and signals through its receptor, the EpoR, on erythroid progenitor cells. Epo binding to EpoR activates the cytosolic tyrosine kinase JAK2, which phosphorylates cytoplasmic tyrosines of the EpoR. Signaling proteins bind these phosphotyrosines through SH2 domains, leading to the survival and proliferation of erythroid progenitor cells and the differentiation of these progenitors into mature erythrocytes.1,2 EpoR signaling is also subject to negative regulation, and the balance between positive and negative regulation allows the system to maintain physiological numbers of circulating red blood cells. Mutations in EpoR or JAK2 that abrogate negative regulation cause erythrocytosis in hematologic malignancies.3,4
A key element in negative regulation of Epo signaling is Epo-dependent endocytosis and degradation of the EpoR.5,6 This process also controls cellular Epo sensitivity and clears Epo from the circulation.7,8 We previously showed that the p85 regulatory subunit of PI3K, by binding to 3 EpoR cytoplasmic tyrosine residues (Y429, Y431, and Y479) phosphorylated by JAK2, is an essential mediator of Epo-induced EpoR internalization via a PI3K kinase activity–independent mechanism.5 This mechanism is physiologically relevant because truncated EpoRs from patients with primary familial and congenital polycythemia (PFCP) lack the 3 key tyrosines, do not bind p85, and cannot be internalized upon Epo stimulation.3,5 PFCP erythroid progenitors are hypersensitive to Epo.9 Appending the pentapeptide KY429LY431L that includes p85-binding sites to a PFCP EpoR rescues receptor internalization and downregulation and normalizes signaling in erythroid progenitor cells.5 How p85 mediates EpoR internalization is not understood.
Ubiquitination plays an important role in the endocytosis of many surface receptors.10-12 Ubiquitination involves the covalent attachment of the protein ubiquitin (Ub) to lysine residues of target proteins. In general, ubiquitination is controlled by a 3-step reaction initialized by E1-mediated Ub activation, followed by conjugation to an E2 enzyme and, finally, substrate targeting by an E3 ligase. The attachment of one Ub molecule to target proteins, mono-ubiquitination, mediates protein trafficking and intracellular signaling.13 Additional Ub molecules can also be added to lysine residues in the previously attached Ub, forming Ub chains. Poly-ubiquitination can signal proteins for degradation.14 Ubiquitinated proteins interact with other proteins containing ubiquitin-binding domains such as ubiquitin-interacting motif (UIM) to form complex signaling networks.15
Casitas B-lineage lymphoma (Cbl, also known as c-Cbl), is an E3 ligase that plays a crucial role in endocytic downregulation of receptor tyrosine kinases.16,17 Cbl contains an N-terminal tyrosine kinase–binding (TKB) domain, followed by a linker and a RING finger domain. The linker critically regulates Cbl ligase activity,18 and mutations in the linker such as deletion of Y368 (ΔY368) or Y371 (ΔY371) abolish Cbl ligase activity and result in cell transformation.19 Cbl was originally identified as the cellular counterpart of v-Cbl that causes myeloid leukemia and lymphomas in mice,20 and Cbl mutations are recently implicated in human myeloid leukemia and neoplasms.16 It was also noted that Cbl−/− mice exhibit erythroid hyperplasia and extramedullary hematopoiesis.21,22 A functional linkage between EpoR and Cbl is suggested by the ability of Epo to induce phosphorylation of Cbl.23,24 The contribution of Cbl to erythropoiesis is not known.
Epsin-1 is an endocytic adaptor protein that promotes clathrin-mediated endocytosis. Through its N-terminal ENTH (Epsin N-terminal homology) domain, it binds to PtdIns(4,5)P2 and induces membrane curvature.25 Importantly, epsin-1 contains binding sites for clathrin and AP-2, as well as 3 UIMs to bind ubiquitinated cargos.26,27 Therefore, epsin-1 links ubiquitinated cargos to the endocytic machinery.
In this study, we determined the molecular mechanism underlying p85-mediated EpoR internalization upon Epo induction. We show that Epo stimulates ubiquitination of p85 via Cbl, and that ubiquitinated p85 recruits epsin-1, thereby inducing EpoR internalization and downregulation. We also show that mutated EpoRs mimicking those from PFCP patients fail to activate this pathway. These studies identify a novel molecular mechanism that drives Epo-induced EpoR endocytosis.
Methods
Plasmid constructs, cell lines, and reagents
γ2A and BaF3 cells stably expressing wild-type or mutant HA-EpoRs were generated as previously described.28 p85α and p85β function redundantly to mediate Epo-induced EpoR internalization,5 so we focused on characterizing p85β in this study and will refer to p85β as p85 henceforth. C-terminal biotinylation–tagged p85 constructs were engineered in the pEBB-TB vector.29 p85 fragments were generated by site-directed mutagenesis or conventional polymerase chain reaction and subcloning. Cbl and its mutants in the pSRα vector were kind gifts of Dr. Wallace Langdon. Cbl−/− mouse embryonic fibroblasts (MEFs) were kind gifts of Dr. Brian Druker. Epsin-1 and epsin-1(ΔUIM) tagged with enhanced cyan fluorescent protein (ECFP) were obtained from Addgene. Recombinant Flag-Ub and Ub(KR) were obtained from Boston Biochem. Antibodies were obtained from the following sources: mouse anti-HA (Covance); JAK2, phospho-JAK2, phospho-tyrosine antibody 4G10 (Millipore); actin and Flag (Sigma); streptavidin agarose (Thermo Scientific); streptavidin-HRP (Biolegend); Cbl (BD Biosciences); rabbit anti-HA, pY731 Cbl, Alexa Fluor 488 conjugated anti-myc and glyceraldehyde 3-phosphate dehydrogenase (GAPDH); cell signaling; p85β and epsin-1 (Santa Cruz); anti-ubiquitinated proteins antibody FK2-conjugated beads (MBL); and T7 tag (Novagen). Anti–green fluorescent protein (GFP) antibody that also recognizes CFP was a gift from Dr. Joachim Seemann. Horseradish peroxidase–coupled secondary antibodies and the ECL chemiluminescence system were from Amersham Biosciences.
Flow cytometry and data analysis
Internalization of surface EpoR was measured by flow cytometry and analyzed as described previously.5 Data were acquired on BD FACSCalibur and analyzed by Flow Jo 8.8.4.
Immunoprecipitation and immunoblotting
γ2A/HA-EpoR/JAK2 cells were transfected with different p85 constructs and induced with Epo. Cell lysates were immunoprecipitated with antibodies as indicated. Under nondenaturing conditions, cells were lysed with 1% NP-40 lysis buffer with phosphatase and protease inhibitors. Under denaturing conditions, cells were lysed with buffer containing 6M urea or 1% sodium dodecyl sulfate (SDS) at 65°C or 100°C for 10 minutes, which was subsequently diluted with NP-40 lysis buffer before immunoprecipitation with FK2 beads. Immunoprecipitation and immunoblotting were performed as described.5 Cell lysates were also immunoblotted with antibodies to HA, phosphorylated JAK2, JAK2, p85, phosphorylated Cbl, Cbl, GAPDH, or actin as indicated.
Cbl knockdown
siRNAs for Cbl (SMART pool ON-TARGET plus L-003003-00-0005) or control siRNA (ON-Target plus D-0011810-01-05) were obtained from Dharmacon. Forty-eight hours after transfection with DharmaFect (Thermo Scientific), cells were induced with Epo and assayed for EpoR internalization. Retroviral shRNA vectors were generated in pWH99 that also expresses GFP (kindly provided by Dr. Harvey Lodish). The hairpin sequence targeting Cbl is: ACCCAGGAACAATATGAATTA, and control shRNA is: CAACAAGATGAAGAGCACCAA. BaF3 cells were co-transduced with 2 retroviral vectors: one expresses Cbl shRNA or a control shRNA with GFP, and the other expresses HA-EpoR with CD4. Cells positive for both GFP and CD4 were isolated via flow cytometry sorting, and Epo-induced EpoR internalization and MTT assays were performed. To determine Epo sensitivity in Ter119− erythroid progenitors, cells were transduced with retroviruses expressing shRNA against Cbl or control shRNA and cultured in expansion conditions (described later). At the indicated days, cell viability was determined by MTT assays.
In vitro ubiquitination assay
Recombinant polyhistidine-tagged Cbl protein was purified from BL21 bacteria via Ni-NTA agarose (Invitrogen). T7-tagged p85 proteins immunoprecipitated from HEK293T cells on Protein A beads were incubated with recombinant Cbl in a ubiquitination reaction mixture containing E1, E2, and Flag-tagged wild-type Ub or lysine-less Ub(KR) (E2 is UbcH5B, ubiquitination buffer is 25 mM Tris-HCl pH 7.6, 5 mM MgCl2, 100 mM NaCl, 1 mM DTT, 2 mM adenosine triphosphate, and 10 mM N-ethylmaleimide (Auto-ubiquitination kit, Enzo Life Sciences) at 37°C for 1 hour. After extensive washing, p85 on Protein A resin was spun down and eluted by SDS sample buffer, run on SDS-polyacrylamide gel electrophoresis (PAGE), and probed with anti-Ub, anti-Flag, or anti-p85 antibodies.
In vitro kinase assay
Cbl immunoprecipitated from HEK293T cells was incubated with glutathione agarose of GST-tagged wild-type or kinase-deficient JAK2 kinase domain in a kinase assay as described.30
Immunofluorescence
γ2A cells stably expressing HA-EpoR, HA-EpoR(5KR), HA-EpoR(PFCP), or HA-EpoR(Rescue) with JAK2 were seeded on glass coverslips. Coverslips were blocked and stained with rabbit anti-HA antibodies before Epo induction. Subsequently, cells were fixed, permeabilized, and incubated with the indicated antibodies. Coverslips were incubated with the appropriate fluorophore-conjugated secondary antibodies and were mounted onto slides with Mowiol mounting medium (Calbiochem). Fluorescence images were taken on a Leica TCS SP5 or Zeiss LSM510 confocal microscope with a 63X oil objective with numeric apertures of 1.25 or 1.4. Confocal section images were analyzed with ImageJ and Adobe Photoshop software. Mander’s coefficients were calculated using the WCIF Intensity Correlation Analysis plug-in in ImageJ.
Isolation of erythroid progenitor cells
Ter119− erythroid progenitors were isolated from E13.5-E14.5 Balb/c murine fetal livers as described.5 For the endogenous p85 ubiquitination experiment, Ter119– cells were expanded in StemPro34 medium with Epo, stem cell factor, insulinlike growth factor-1, and dexamethasone for 3 days.31 The cells were starved for 4 hours and induced with Epo at indicated time points. For immunofluorescence, Ter119– cells expressing HA-EpoR were seeded on poly-l-lysine–coated coverslips and starved before immunostaining, as described before.
MTT assay
10 000 BaF3 cells and 30 000 primary erythroid progenitors were seeded per well of a 96-well plate, and MTT assay was performed (Promega).
Results
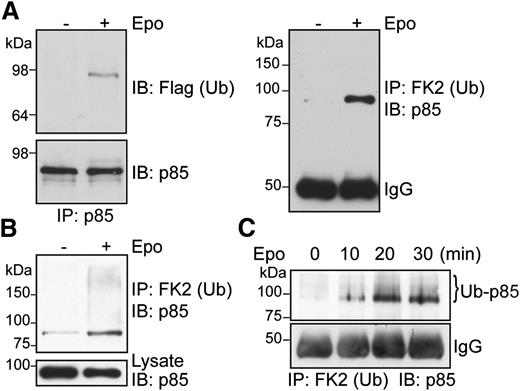
p85 becomes ubiquitinated upon Epo stimulation
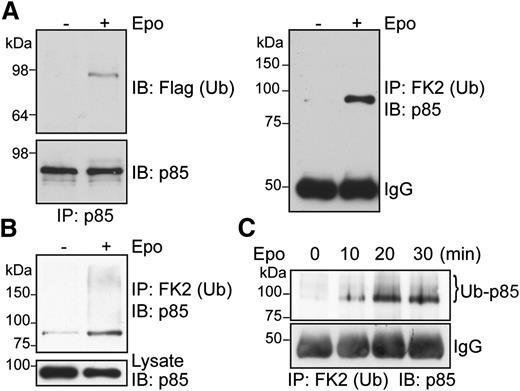
We showed previously that p85 mediates Epo-induced EpoR internalization in a PI3K kinase activity–independent manner, but the mechanism is not clear. We discovered that p85 becomes ubiquitinated upon Epo stimulation in γ2A/HA-EpoR/JAK2 cells that stably express HA-tagged EpoR and JAK2. Ubiquitinated p85 was detected in these cells transiently expressing p85 and Flag-tagged ubiquitin (Flag-Ub) by immunoprecipitation with anti-p85 antibodies and immunoblotting with anti-Flag antibodies (Figure 1A, left), and also by immunoprecipitation with anti-ubiquitinated proteins antibody (FK2) and immunoblotting with anti-p85 antibodies (Figure 1A, right). To eliminate the possibility that a p85-associated protein of similar molecular weight becomes ubiquitinated upon Epo stimulation, we performed immunoprecipitation with FK2 antibody under denaturing conditions followed by immunoblotting with anti-p85 antibodies. Similar to previous results, Epo-induced ubiquitination of p85 was observed (Figure 1B). On the basis of the apparent molecular weight on SDS-PAGE, p85 likely is mono-ubiquitinated upon Epo stimulation. However, some higher-molecular-weight–ubiquitinated p85 species (seen as a high-molecular-weight smear) were also detected under denaturing conditions. Importantly, we verified our results on endogenous p85 protein in the physiologically relevant primary erythroid progenitors. Erythroid progenitors can be isolated from E13.5-14.5 murine fetal livers by depletion of Ter119+ cells.5,32 These Ter119− erythroid progenitors are highly Epo-responsive and can undergo terminal differentiation in vitro into reticulocytes. We expanded these cells in media containing Epo, stem cell factor, insulinlike growth factor-1, and dexamethasone to keep them in an undifferentiated state as described.31 These cells were washed, starved for 4 hours, and stimulated with Epo, and ubiquitinated proteins were immunoprecipitated by FK2 antibody under denaturing conditions and immunoblotted with anti-p85 antibodies. Ubiquitination of endogenous p85 was readily detectable as early as 10 minutes after Epo stimulation, and the amount of ubiquitinated p85 reached a stable level 20 minutes after Epo stimulation (Figure 1C). Therefore, consistent with our results in cell lines, Epo-induced p85 ubiquitination was observed in primary erythroid progenitors. We surmised that ubiquitinated p85 may serve as an endocytic signal to internalize EpoR upon Epo stimulation.
p85 becomes ubiquitinated upon Epo stimulation. (A) γ2A/HA-EpoR/JAK2 cells transiently expressing p85 and Flag-tagged Ub were stimulated with Epo for 15 minutes. Immunoprecipitated p85 under nondenaturing conditions was blotted with the indicated antibodies. (B) Ubiquitinated p85 was also detected by immunoprecipitation using FK2 antibody–conjugated beads under denaturing conditions and immunoblotting for p85. (C) Epo induces endogenous p85 ubiquitination in primary Ter119− erythroid progenitor cells. FK2, anti-ubiquitinated proteins antibody; IP, immunoprecipitation; IB, immunoblot.
p85 becomes ubiquitinated upon Epo stimulation. (A) γ2A/HA-EpoR/JAK2 cells transiently expressing p85 and Flag-tagged Ub were stimulated with Epo for 15 minutes. Immunoprecipitated p85 under nondenaturing conditions was blotted with the indicated antibodies. (B) Ubiquitinated p85 was also detected by immunoprecipitation using FK2 antibody–conjugated beads under denaturing conditions and immunoblotting for p85. (C) Epo induces endogenous p85 ubiquitination in primary Ter119− erythroid progenitor cells. FK2, anti-ubiquitinated proteins antibody; IP, immunoprecipitation; IB, immunoblot.
Epo induces p85 binding to Cbl
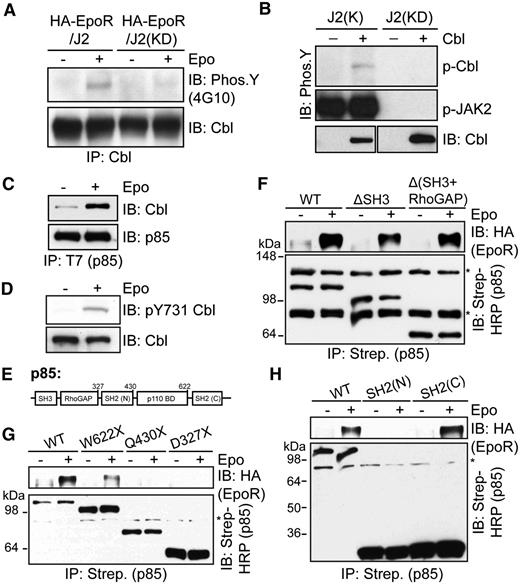
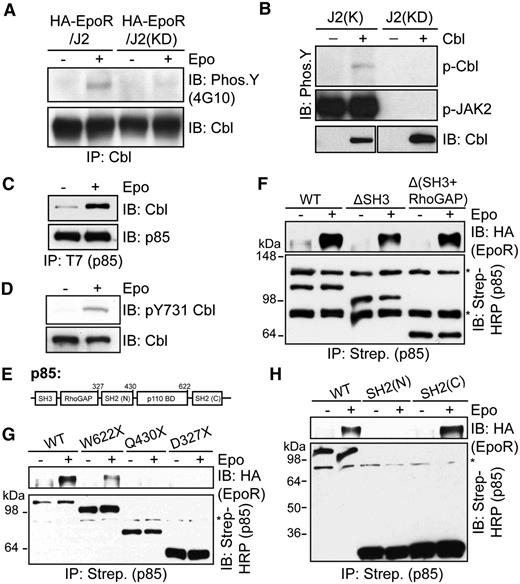
The E3 ligase Cbl, which mediates ubiquitination and endocytosis of multiple receptor tyrosine kinases, was previously shown to bind p85 in stimulated B lymphocytes and in Jurkat T cells.33,34 In addition, erythroid progenitors appeared to have expanded in Cbl−/− mice.21 We thus examined the possibility that Cbl is the E3 ligase that ubiquitinates p85 upon Epo stimulation. Epo induced tyrosine phosphorylation of Cbl in γ2A/HA-EpoR/JAK2 cells, similar to previous reports, but not γ2A/HA-EpoR/JAK2KD cells, which express kinase-deficient JAK2 (Figure 2A).23,24 Cbl phosphorylation is likely mediated by JAK2 because we observed that JAK2 could directly phosphorylate Cbl in in vitro kinase assays (Figure 2B). Importantly, we found that Epo induced p85 binding to Cbl (Figure 2C). Consistent with previous findings that the N-terminal SH2 domain of p85 binds to Cbl Y731 in the predicted p85-binding motif (Y731EAM),35-39 Epo induced phosphorylation of Cbl Y731 (Figure 2D).
Epo induces p85 interaction with Cbl. (A) Cbl becomes tyrosine-phosphorylated upon Epo stimulation in γ2A/HA-EpoR cells expressing wild-type JAK2 but not kinase-deficient (KD) JAK2. Cell lysates were subjected to immunoprecipitation by Cbl antibodies and immunoblotted with 4G10 to detect phospho-tyrosine. (B) JAK2 phosphorylates Cbl in vitro. Cbl proteins immunoprecipitated from HEK293T cells were incubated with GST-tagged wild-type or kinase-deficient JAK2 kinase domain in in vitro kinase assay. J2(K), JAK2 kinase domain; J2(KD), kinase-deficient JAK2 kinase domain. (C) Epo induces p85 interaction with Cbl. γ2A/HA-EpoR/JAK2 cells transiently expressing Cbl and T7-tagged p85 were induced with Epo. p85 was immunoprecipitated with T7 antibodies, and the immunoprecipitates were probed with antibodies against Cbl. (D) Epo induces phosphorylation of Y731 in the p85-binding motif of Cbl. (E) p85 domain structure. SH3, src-homology 3 domain; RhoGAP, RhoGAP homology domain; SH2, src-homology 2 domain; p110BD, p110 binding domain. (F-H) The C-terminal SH2 domain of p85 mediates inducible binding to EpoR upon stimulation. Biotin-tagged p85 fragments were transiently expressed in γ2A/HA-EpoR/JAK2 cells, isolated with streptavidin agarose, and probed with anti-HA to detect bound EpoR. (G) W622X, Q430X, and D327X are truncations where residues W622, Q430, or D327 were mutated to a stop codon. IP, immunoprecipitation; IB, immunoblot. *Nonspecific bands.
Epo induces p85 interaction with Cbl. (A) Cbl becomes tyrosine-phosphorylated upon Epo stimulation in γ2A/HA-EpoR cells expressing wild-type JAK2 but not kinase-deficient (KD) JAK2. Cell lysates were subjected to immunoprecipitation by Cbl antibodies and immunoblotted with 4G10 to detect phospho-tyrosine. (B) JAK2 phosphorylates Cbl in vitro. Cbl proteins immunoprecipitated from HEK293T cells were incubated with GST-tagged wild-type or kinase-deficient JAK2 kinase domain in in vitro kinase assay. J2(K), JAK2 kinase domain; J2(KD), kinase-deficient JAK2 kinase domain. (C) Epo induces p85 interaction with Cbl. γ2A/HA-EpoR/JAK2 cells transiently expressing Cbl and T7-tagged p85 were induced with Epo. p85 was immunoprecipitated with T7 antibodies, and the immunoprecipitates were probed with antibodies against Cbl. (D) Epo induces phosphorylation of Y731 in the p85-binding motif of Cbl. (E) p85 domain structure. SH3, src-homology 3 domain; RhoGAP, RhoGAP homology domain; SH2, src-homology 2 domain; p110BD, p110 binding domain. (F-H) The C-terminal SH2 domain of p85 mediates inducible binding to EpoR upon stimulation. Biotin-tagged p85 fragments were transiently expressed in γ2A/HA-EpoR/JAK2 cells, isolated with streptavidin agarose, and probed with anti-HA to detect bound EpoR. (G) W622X, Q430X, and D327X are truncations where residues W622, Q430, or D327 were mutated to a stop codon. IP, immunoprecipitation; IB, immunoblot. *Nonspecific bands.
We also determined p85 domain(s) required for binding to EpoR upon Epo stimulation using constructs of individual p85 domains fused to a biotinylation sequence, which is biotinylated by the endogenous mammalian machinery, to allow isolation via streptavidin agarose (Figure 2E-H). The SH3 and RhoGAP homology domains were dispensable for EpoR binding (Figure 2F). Consistent with the fact that p85 binds to phosphotyrosines on the EpoR, W622X lacking the C-terminal SH2 domain had greatly reduced binding to EpoR (Figure 2G). Conversely, the C-terminal SH2 domain alone, but not the N-terminal SH2 domain, was sufficient to bind to EpoR (Figure 2H). Therefore, the C-terminal SH2 domain of p85 likely mediates EpoR binding. The p110-binding domain also somehow contributed to the p85-EpoR interaction, because the residual binding of W622X to EpoR was abolished in Q430X (Figure 2G). Together, these results suggested that upon Epo stimulation, the C-terminal SH2 domain of p85 binds to phosphorylated Y429, Y431, or Y479 of EpoR. In addition, Cbl Y731 becomes phosphorylated upon Epo stimulation, facilitating Cbl binding to p85.
Cbl and its E3 ligase activity are essential for Epo-induced EpoR internalization
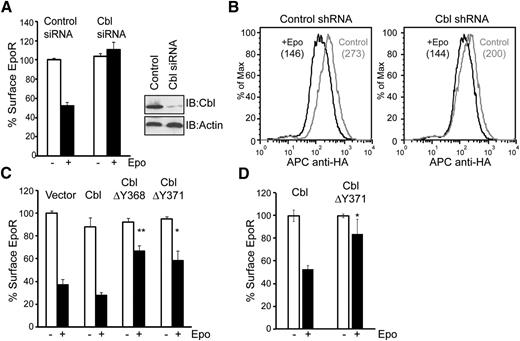
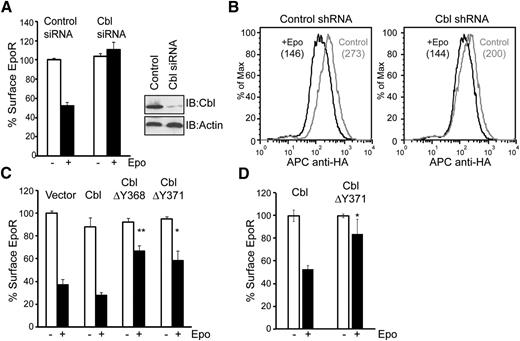
We examined whether Cbl contributes to Epo-induced EpoR internalization. We used siRNAs to knockdown Cbl in γ2A/HA-EpoR/JAK2 cells. As shown in Figure 3A, Epo-induced EpoR internalization was blocked in Cbl knockdown cells compared with cells transfected with control siRNAs, measured using anti-HA antibodies to label the exofacial HA-tag of EpoR. We also determined whether Cbl contributes to Epo-induced EpoR internalization in hematopoietic BaF3 cells. BaF3 cells were transduced with 2 retroviral vectors, one expressing Cbl shRNA or a control shRNA and GFP, and the other expressing HA-EpoR and human CD4 after an internal ribosomal entry site. Cells transduced with both vectors were isolated via flow cytometry sorting and examined for Epo-induced EpoR internalization. As shown in Figure 3B, Epo-induced EpoR internalization was mildly but significantly impaired in cells expressing Cbl shRNA compared with control cells, possibly because of modest knockdown efficiency (supplemental Figure 1).
Cbl and its E3 ligase activity are essential for Epo-induced EpoR internalization. (A) siRNAs for Cbl or control siRNAs were transfected into γ2A/HA-EpoR/JAK2 cells, and levels of surface EpoR before and after 45 minutes of Epo stimulation were analyzed by flow cytometry. For each sample, EpoR surface expression was normalized to that from samples expressing control siRNA before Epo stimulation. Knockdown efficiency of Cbl is shown. Immunoblotting of actin was used as a loading control. (B) Cbl knockdown in BaF3 cells impairs Epo-induced EpoR internalization. Cells expressing shRNA for Cbl or control shRNA, identified by GFP expression, were gated for analyses. The numbers in parentheses are median fluorescence intensity, which represents EpoR surface expression. Representative histograms from 3 independent experiments are shown. (C) Epo-induced EpoR internalization is impaired in γ2A/HA-EpoR/JAK2 cells expressing ligase-deficient Cbl mutants (ΔY368 and ΔY371). (D) Epo-induced EpoR internalization is significantly impaired by the expression of dominant-negative Cbl in Ter119– erythroid progenitor cells. *P < .05, **P < .005 (Student t test).
Cbl and its E3 ligase activity are essential for Epo-induced EpoR internalization. (A) siRNAs for Cbl or control siRNAs were transfected into γ2A/HA-EpoR/JAK2 cells, and levels of surface EpoR before and after 45 minutes of Epo stimulation were analyzed by flow cytometry. For each sample, EpoR surface expression was normalized to that from samples expressing control siRNA before Epo stimulation. Knockdown efficiency of Cbl is shown. Immunoblotting of actin was used as a loading control. (B) Cbl knockdown in BaF3 cells impairs Epo-induced EpoR internalization. Cells expressing shRNA for Cbl or control shRNA, identified by GFP expression, were gated for analyses. The numbers in parentheses are median fluorescence intensity, which represents EpoR surface expression. Representative histograms from 3 independent experiments are shown. (C) Epo-induced EpoR internalization is impaired in γ2A/HA-EpoR/JAK2 cells expressing ligase-deficient Cbl mutants (ΔY368 and ΔY371). (D) Epo-induced EpoR internalization is significantly impaired by the expression of dominant-negative Cbl in Ter119– erythroid progenitor cells. *P < .05, **P < .005 (Student t test).
To determine whether E3 ligase activity of Cbl is required for EpoR internalization, we examined the effect of 2 ligase-deficient Cbl mutants, ΔY368 and ΔY371, on Epo-induced EpoR internalization.19,40 As is shown in Figure 3C, Cbl(ΔY368) and Cbl(ΔY371) both had a dominant-negative effect on the internalization of EpoR in γ2A/HA-EpoR/JAK2 cells, whereas expression of wild-type Cbl had no effect, demonstrating that Cbl E3 ligase activity is critical for Epo-induced EpoR internalization. Importantly, these results were verified in Ter119– erythroid progenitors (Figure 3D). Together, our results showed that Epo induces p85 ubiquitination and its interaction with Cbl, and that Cbl and its ligase activity play a crucial role in Epo-induced EpoR internalization.
Cbl ubiquitinates p85 in vitro
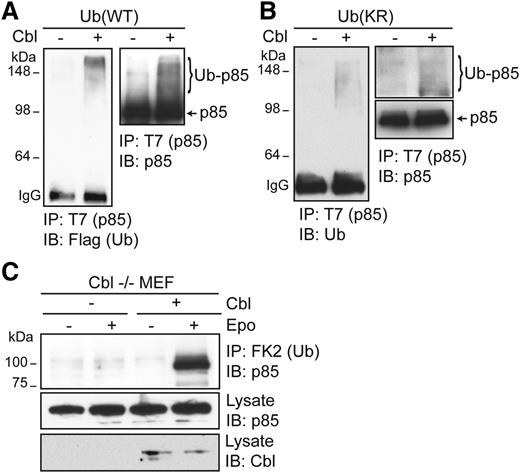
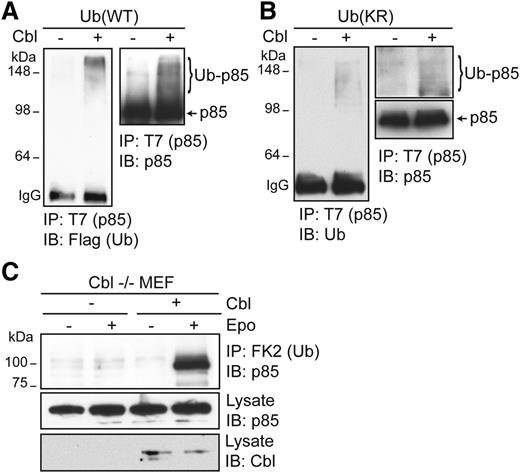
To corroborate our results, we examined whether Cbl can directly ubiquitinate p85 using an in vitro ubiquitination assay. Incubation of recombinant Cbl and Flag-Ub proteins with T7-tagged p85, immunoprecipitated from HEK293T cells, in in vitro ubiquitination assays resulted in the appearance of higher-molecular-weight ubiquitinated p85 species, detectable by immunoblotting with anti-Flag or anti-p85 antibodies. In contrast, these species were not detected in the absence of Cbl (Figure 4A). To determine the nature of ubiquitination on p85, we also examined p85 ubiquitination with a lysine-less Ub mutant that cannot form ubiquitin chains and only supports mono-ubiquitination (Ub[KR]). In reactions with recombinant Ub(KR), high-molecular-weight–ubiquitinated p85 species were still detected, indicating that Cbl can ubiquitinate p85 on multiple sites in vitro (Figure 4B). We also examined p85 ubiquitination in cells from Cbl−/− animals. Cbl−/− MEFs were immortalized and transfected with vectors expressing HA-EpoR, JAK2, p85, and Flag-Ub. Epo-induced p85 ubiquitination was not observed in Cbl−/− MEFs, and re-expression of Cbl in these cells rescued Epo-induced p85 ubiquitination (Figure 4C). These results demonstrate that p85 is ubiquitinated by Cbl upon Epo stimulation.
Cbl ubiquitinates p85 in vitro. (A) T7-tagged p85 immunoprecipitated from HEK293T cells using anti-T7 antibody and Protein A beads was incubated with 200 ng of recombinant Cbl purified from BL21 in in vitro ubiquitination assay using recombinant E1, E2 (UbcH5B), and Flag-tagged wild-type Ub. After extensive washing, ubiquitinated p85 species were eluted from Protein A beads by SDS sample buffer and immunoblotted with the indicated antibodies. (B) p85 can be ubiquitinated at multiple sites. An in vitro ubiquitination assay was performed with a lysine-less ubiquitin mutant (KR) that cannot form ubiquitin chains. (C) p85 ubiquitination is lost in Cbl−/− MEFs and is restored in Cbl−/− MEFs reconstituted with Cbl.
Cbl ubiquitinates p85 in vitro. (A) T7-tagged p85 immunoprecipitated from HEK293T cells using anti-T7 antibody and Protein A beads was incubated with 200 ng of recombinant Cbl purified from BL21 in in vitro ubiquitination assay using recombinant E1, E2 (UbcH5B), and Flag-tagged wild-type Ub. After extensive washing, ubiquitinated p85 species were eluted from Protein A beads by SDS sample buffer and immunoblotted with the indicated antibodies. (B) p85 can be ubiquitinated at multiple sites. An in vitro ubiquitination assay was performed with a lysine-less ubiquitin mutant (KR) that cannot form ubiquitin chains. (C) p85 ubiquitination is lost in Cbl−/− MEFs and is restored in Cbl−/− MEFs reconstituted with Cbl.
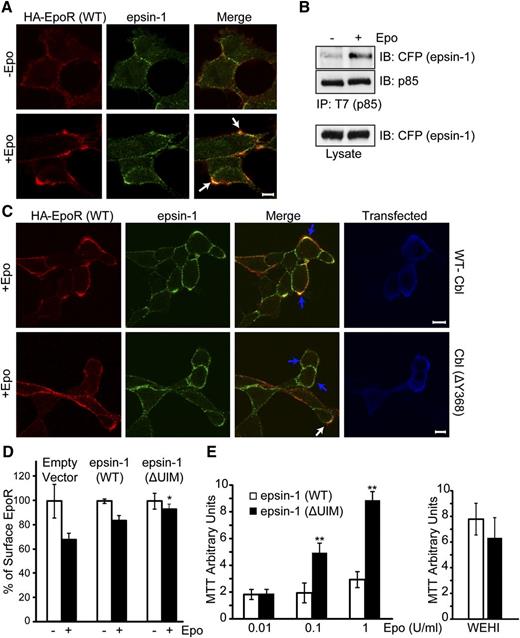
Epo induces co-localization of epsin-1 with the EpoR
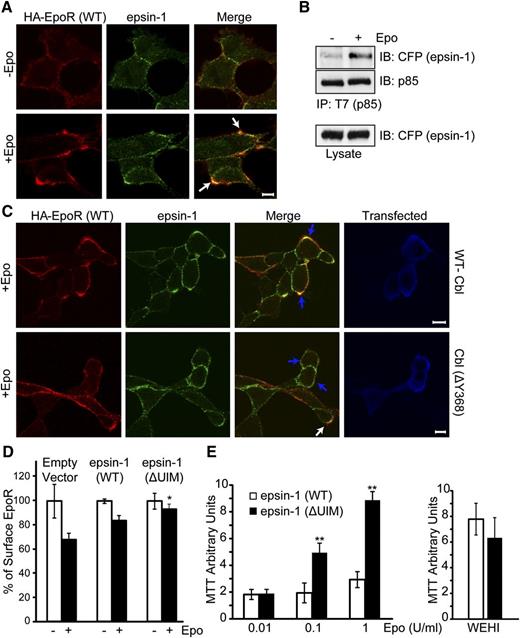
We determined the functional consequence of p85 ubiquitination by Cbl. Because Ub can trigger the internalization of cargos by binding to endocytic ubiquitin–binding proteins,41 we examined candidate endocytic proteins known to bind ubiquitinated cargos and tested whether they become co-localized with EpoR upon Epo stimulation. We found that epsin-1, which couples ubiquitinated cargos through its ubiquitin-interacting motifs (UIMs) to clathrin and AP-2,25 co-localized with the EpoR upon Epo induction (Figure 5A, larger panel of cells with Mander’s overlap coefficients in supplemental Figure 2A). Consistent with these results, Epo induced association between p85 and epsin-1 tagged with ECFP (Figure 5B). To rule out that epsin-1 is recruited to EpoR itself, which is also ubiquitinated upon Epo stimulation, we examined a lysine-less EpoR mutant that cannot be ubiquitinated (HA-EpoR[5KR]).42 HA-EpoR(5KR), similar to wild-type HA-EpoR, co-localized with epsin-1 upon Epo stimulation (supplemental Figure 2B), suggesting that recruitment of epsin-1 to EpoR does not require a ubiquitin moiety on EpoR itself and may instead bind to ubiquitinated p85. We also tested the effect of expressing a ligase-deficient Cbl mutant on co-localization of EpoR with epsin-1. As shown in Figure 5C (also supplemental Figure 3), cells expressing wild-type myc-tagged Cbl (identified by Alexa488-conjugated anti-myc) exhibited co-localization (blue arrows, upper merge panel), similar to nonexpressing cells. However, Cbl(ΔY368)-expressing cells showed greatly reduced EpoR co-localization with epsin-1 (blue arrows, lower merge panel). As controls, co-localization between EpoR and epsin-1 was normal in untransfected cells (white arrow, lower merge panel). We also tested whether EpoR co-localizes with endosomal markers upon longer Epo treatment. Both γ2A/HA-EpoR/JAK2 and Ter119– progenitors exhibited co-localization of HA-EpoR with early endosome antigen EEA1 upon 25 minutes of Epo induction (supplemental Figure 4).
Epo induces EpoR co-localization with epsin-1. (A) Endogenous epsin-1 becomes co-localized with wild-type EpoR upon Epo stimulation. Nonpermeabilized γ2A/HA-EpoR/JAK2 cells were stained with anti-HA antibodies to label the exofacial HA-tag of the EpoR as described previously.5 Cell were then treated with Epo for 12 minutes, fixed, permeabilized, immunostained for epsin-1, and visualized by confocal microscopy. The scale bar represents 5 μm. Representative co-localization areas are marked with arrows. (B) Epo induces p85 binding to epsin-1. ECFP-tagged epsin-1 and T7-tagged p85 were transiently expressed in γ2A/HA-EpoR/JAK2. Anti-T7 immunoprecipitates were immunoblotted with anti-GFP antibody to detect epsin-1. (C) EpoR-epsin-1 co-localization is impaired in cells expressing a ligase-deficient Cbl mutant. γ2A/HA-EpoR/JAK2 cells were transfected with either wild-type myc-tagged Cbl or Cbl(ΔY368). Forty-eight hours after transfection, cells were stained as described before, except Alexa 488 anti-myc antibody was used to mark transfected cells. The blue arrows indicate the transfected cells, and the white arrow shows co-localization in an untransfected cell. (D) Expression of epsin-1(ΔUIM) impaired EpoR internalization. Epo-induced EpoR internalization was determined by flow cytometry in γ2A cells co-transfected with vectors expressing HA-EpoR, JAK2, and ECFP-tagged epsin-1 (either wild-type or the ΔUIM mutant). Data were normalized to EpoR surface expression of unstimulated control cells. *P < .05 between epsin-1(WT) and epsin-1(ΔUIM). (E) BaF3 cells expressing HA-EpoR and epsin-1 (either wild-type or the ΔUIM mutant) were grown in RPMI media containing 2% fetal bovine serum (FBS) with different concentrations of Epo. Cell growth was measured using MTT assays. Cells grew similarly in WEHI media and 10% FBS. Epsin-1(ΔUIM)–expressing cells exhibit Epo hypersensitivity. **P < .005.
Epo induces EpoR co-localization with epsin-1. (A) Endogenous epsin-1 becomes co-localized with wild-type EpoR upon Epo stimulation. Nonpermeabilized γ2A/HA-EpoR/JAK2 cells were stained with anti-HA antibodies to label the exofacial HA-tag of the EpoR as described previously.5 Cell were then treated with Epo for 12 minutes, fixed, permeabilized, immunostained for epsin-1, and visualized by confocal microscopy. The scale bar represents 5 μm. Representative co-localization areas are marked with arrows. (B) Epo induces p85 binding to epsin-1. ECFP-tagged epsin-1 and T7-tagged p85 were transiently expressed in γ2A/HA-EpoR/JAK2. Anti-T7 immunoprecipitates were immunoblotted with anti-GFP antibody to detect epsin-1. (C) EpoR-epsin-1 co-localization is impaired in cells expressing a ligase-deficient Cbl mutant. γ2A/HA-EpoR/JAK2 cells were transfected with either wild-type myc-tagged Cbl or Cbl(ΔY368). Forty-eight hours after transfection, cells were stained as described before, except Alexa 488 anti-myc antibody was used to mark transfected cells. The blue arrows indicate the transfected cells, and the white arrow shows co-localization in an untransfected cell. (D) Expression of epsin-1(ΔUIM) impaired EpoR internalization. Epo-induced EpoR internalization was determined by flow cytometry in γ2A cells co-transfected with vectors expressing HA-EpoR, JAK2, and ECFP-tagged epsin-1 (either wild-type or the ΔUIM mutant). Data were normalized to EpoR surface expression of unstimulated control cells. *P < .05 between epsin-1(WT) and epsin-1(ΔUIM). (E) BaF3 cells expressing HA-EpoR and epsin-1 (either wild-type or the ΔUIM mutant) were grown in RPMI media containing 2% fetal bovine serum (FBS) with different concentrations of Epo. Cell growth was measured using MTT assays. Cells grew similarly in WEHI media and 10% FBS. Epsin-1(ΔUIM)–expressing cells exhibit Epo hypersensitivity. **P < .005.
To corroborate these results, we examined an epsin-1 mutant lacking its UIMs and thus not able to interact with ubiquitinated cargos (epsin-1[ΔUIM]). Contrary to wild-type epsin-1, epsin-1(ΔUIM) expression impaired Epo-induced EpoR internalization (Figure 5D). Consistent with this defect in EpoR internalization, BaF3/HA-EpoR cells expressing epsin-1(ΔUIM) exhibited increased Epo sensitivity compared with cells expressing wild-type epsin-1 (Figure 5E). Together, our results support a model in which Epo induces binding of EpoR and p85, which is ubiquitinated by Cbl. Ubiquitination allows p85 to recruit epsin-1 via its UIMs to the EpoR/p85 complex and engages the endocytic machinery for EpoR internalization and downregulation (Figure 7C).
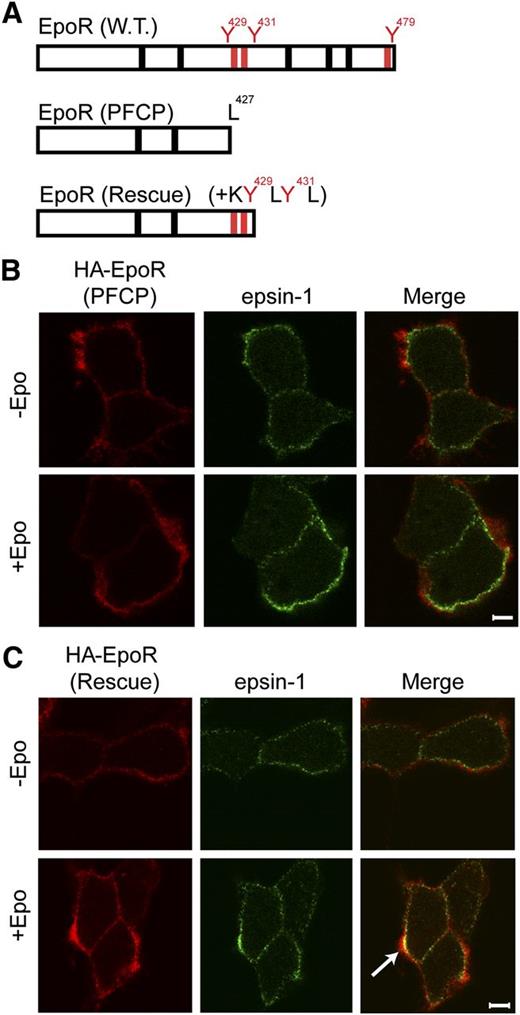
Truncated PFCP EpoR is defective in epsin-1 co-localization upon Epo stimulation
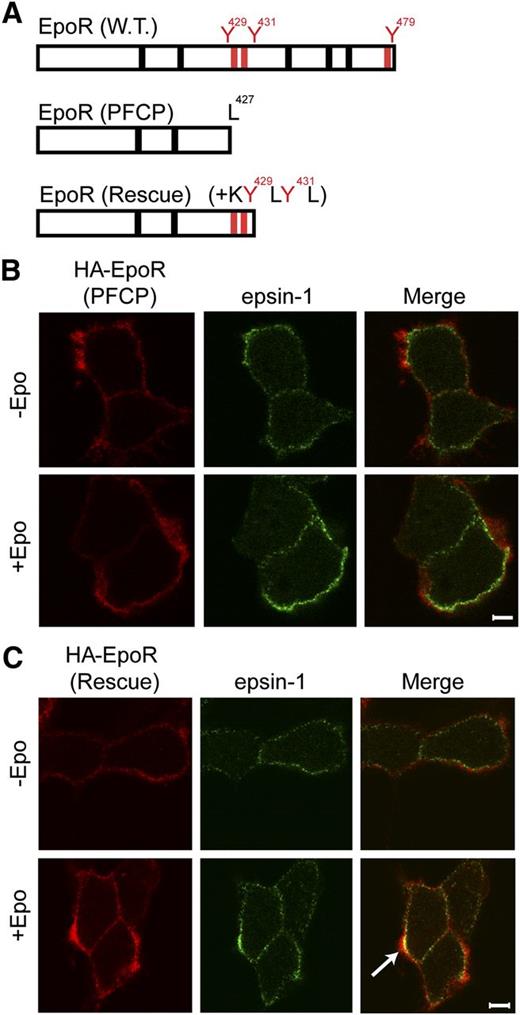
Erythroid progenitors from PFCP patients harbor truncated EpoRs and display Epo hypersensitivity.9 These PFCP receptors lack p85-binding sites.5 We examined the ability of a truncated EpoR that was engineered to mimic a PFCP EpoR allele, HA-EpoR(PFCP), and its rescue counterpart with appended KY429LY431L p85-binding sites, HA-EpoR(Rescue), to recruit epsin-1 upon Epo stimulation (Figure 6A). Consistent with the inability to recruit p85, HA-EpoR(PFCP) failed to co-localize with epsin-1 upon Epo stimulation (Figure 6B, supplemental Figure 5A). Importantly, HA-EpoR(Rescue) co-localized with epsin-1 similar to wild-type HA-EpoR (Figures 6C and 5A and supplemental Figure 5B). Importantly, EpoR internalization was restored and Epo hypersensitivity was normalized in erythroid progenitors expressing HA-EpoR(Rescue) compared with HA-EpoR(PFCP).5
Appendage of p85-binding sites restores Epo-induced epsin-1 co-localization of a truncated EpoR that mimics those from PFCP patients in γ2A cells. (A) Schematic diagram of PFCP and rescue EpoR constructs. (B) PFCP EpoR did not co-localize with epsin-1 upon Epo stimulation. (C) Rescue EpoR appended with p85-binding sites rescued Epo-induced epsin-1 co-localization. The arrow points to a representative co-localization area.
Appendage of p85-binding sites restores Epo-induced epsin-1 co-localization of a truncated EpoR that mimics those from PFCP patients in γ2A cells. (A) Schematic diagram of PFCP and rescue EpoR constructs. (B) PFCP EpoR did not co-localize with epsin-1 upon Epo stimulation. (C) Rescue EpoR appended with p85-binding sites rescued Epo-induced epsin-1 co-localization. The arrow points to a representative co-localization area.
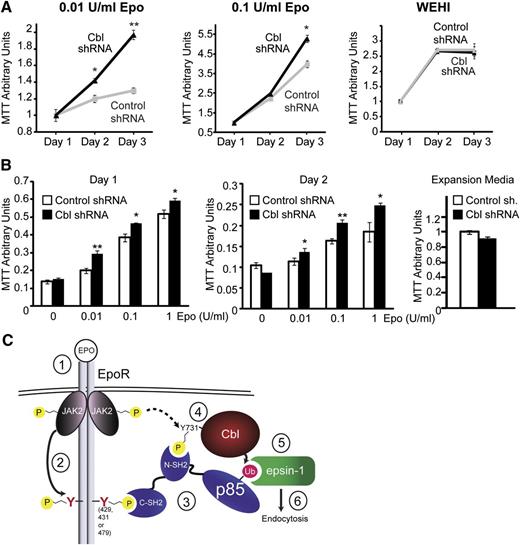
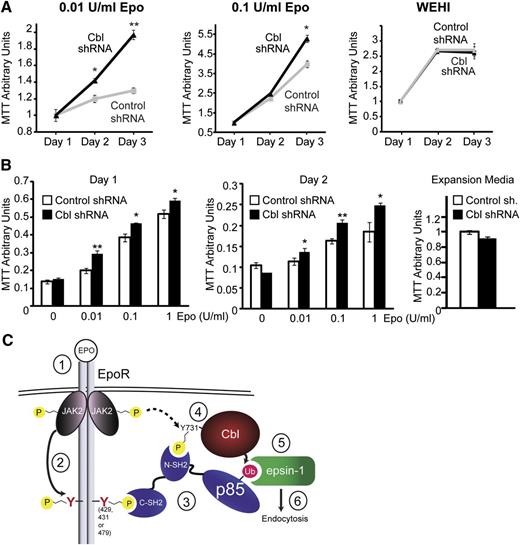
Cbl knockdown increases Epo sensitivity in primary erythroid progenitors
To implicate the physiological relevance of Cbl in EpoR endocytosis and signaling, we knocked down Cbl in hematopoietic BaF3 cells and in primary Ter119– erythroid progenitors and examined their Epo sensitivity. As is shown in Figure 7A, IL-3–dependent BaF3 cells stably expressing HA-EpoR with Cbl shRNA or control shRNA grew similarly in control media containing IL-3 (WEHI conditioned media). In contrast, despite a modest knockdown efficiency and a slightly lower HA-EpoR surface expression level (supplemental Figures 1 and 6), Cbl knockdown BaF3 cells had higher mitogenic activities in media containing 0.1 U/mL and 0.01 U/mL Epo compared with control knockdown cells. Similarly, at comparable transduction efficiency (based on GFP fluorescence, data not shown), Cbl knockdown in Ter119– cells caused Epo hypersensitivity at different Epo concentrations compared with control cells (Figure 7B). Therefore, knockdown of Cbl phenocopied deletion of p85-binding sites from EpoR, as in PFCP EpoRs, and resulted in Epo hypersignaling in primary erythroid progenitors. These results elucidated the physiological relevance of our Cbl/p85/epsin-1 pathway to internalize and downregulate EpoR upon Epo stimulation.
Cbl knockdown results in hypersensitivity to Epo. (A) BaF3 cells expressing HA-EpoR and either Cbl shRNA or control shRNA were grown in RPMI media containing 2% FBS with different concentrations of Epo. Cell growth was measured using MTT assays. Cells grew similarly in control media containing no Epo but IL-3 (WEHI media) and 10% FBS. *P < .05, **P < .005. (B) Expression of Cbl shRNA but not control shRNA in primary Ter119– erythroid progenitors resulted in Epo hypersensitivity in IMDM media containing 2% FBS and different concentrations of Epo. Cells grew similarly in control expansion media (2 U/mL Epo). *P < .05, **P < .005. (C) A working model of p85-mediated EpoR internalization. Epo stimulates binding of p85 to both Cbl and the EpoR through its SH2 domains, possibly forming a tripartite complex. Epo also induces Cbl ubiquitination of p85, which in turn recruits epsin-1 through its UIMs to further enlist the endocytic machinery for EpoR internalization (details are described in the Discussion).
Cbl knockdown results in hypersensitivity to Epo. (A) BaF3 cells expressing HA-EpoR and either Cbl shRNA or control shRNA were grown in RPMI media containing 2% FBS with different concentrations of Epo. Cell growth was measured using MTT assays. Cells grew similarly in control media containing no Epo but IL-3 (WEHI media) and 10% FBS. *P < .05, **P < .005. (B) Expression of Cbl shRNA but not control shRNA in primary Ter119– erythroid progenitors resulted in Epo hypersensitivity in IMDM media containing 2% FBS and different concentrations of Epo. Cells grew similarly in control expansion media (2 U/mL Epo). *P < .05, **P < .005. (C) A working model of p85-mediated EpoR internalization. Epo stimulates binding of p85 to both Cbl and the EpoR through its SH2 domains, possibly forming a tripartite complex. Epo also induces Cbl ubiquitination of p85, which in turn recruits epsin-1 through its UIMs to further enlist the endocytic machinery for EpoR internalization (details are described in the Discussion).
Discussion
In this study, we identified a novel Cbl/p85/epsin-1 pathway by which Epo induces EpoR endocytosis and downregulation. In our model (Figure 7C), upon Epo stimulation, JAK2 (1) becomes activated and (2) phosphorylates EpoR cytoplasmic tyrosine residues, including Y429, Y431, and Y479, which (3) serve as docking sites for SH2 domain-mediated binding of p85 to EpoR. In addition, (4) Cbl Y731 becomes phosphorylated upon Epo stimulation, facilitating Cbl binding to p85. (5) Cbl ubiquitinates p85, enabling recruitment of epsin-1 to ubiquitinated p85 through its UIMs. (6) Binding of the EpoR/p85 complex to epsin-1 connects it to the clathrin-mediated endocytic machinery to induce EpoR internalization. We also showed that mutated EpoRs mimicking those found in PFCP patients can neither bind p85 nor recruit epsin-1 to engage the endocytic machinery, thus they do not internalize upon Epo stimulation and exhibit Epo hypersensitivity. Similarly, knockdown of Cbl also causes Epo hypersensitivity in primary erythroid progenitors. Restoring p85 binding to PFCP receptors rescues Epo-induced epsin-1 co-localization and normalizes Epo hypersensitivity. Our results elucidate the molecular mechanism underlying Epo-induced p85-mediated EpoR internalization and demonstrate that defect in this pathway may contribute to the etiology of PFCP.
Our study identifies a novel PI3K kinase activity–independent function of p85, namely to recruit endocytic protein epsin-1 via its ubiquitination for EpoR internalization. This adds to the emerging concept that p85 has functions in addition to regulating PI3K kinase activity43-46 and represents the first recognition of cytokine receptors as cargos of epsins. Other cytokine receptors could be regulated by similar mechanisms because most cytokine receptors activate PI3K, often through recruitment of p85. Epo-induced p85 ubiquitination appears to be a mono-ubiquitination. Interestingly, because higher-molecular-weight ubiquitinated p85 species were observed under denaturing cell lysis conditions, and because Cbl ubiquitinates multiple sites on p85 in vitro, it is possible that a de-ubiquitinating enzyme is involved in this process. However, thus far, no de-ubiquitinating enzyme has been implicated in EpoR signaling.
We found that Cbl is an essential mediator in Epo-induced EpoR downregulation, and p85 is a novel Cbl substrate. This is consistent with the notion that Cbl constrains signal transduction for surface receptors. Cbl-b, another member in the Cbl family, was also reported to ubiquitinate p85. In that case, proteolysis-independent poly-ubiquitination of p85 affects its recruitment to CD28 or T-cell antigen receptor ζ.47 Ubiquitination via different E3 Ub ligases regulates multiple steps in the process of EpoR endocytosis. In addition to Cbl that mediates Epo-induced EpoR internalization via p85 ubiquitination, β-Trcp (β-transducin repeat–containing protein) was shown to mediate ubiquitination of EpoR itself and facilitate its degradation.42,48 These steps may cooperatively ensure the proper downregulation of EpoR upon stimulation.
Walrafen et al showed that proteosomal degradation removes a portion of the EpoR cytoplasmic domain that contains the tyrosine residues before the receptor is internalized.6 In published work, we too noted tyrosine-independent EpoR internalization at high Epo concentration,5 but the contribution of this mechanism was small relative to the tyrosine-dependent pathway. Multiple EpoR internalization mechanisms may exist depending on physiological contexts.
Recently, Cbl mutants have been found in human myeloid leukemias and neoplasms.16 Interestingly, studies of these mutants implicated that Cbl may possess both tumor suppressor and oncogenic functions. However, underlying mechanisms and their dependency on Cbl’s E3 ubiquitin ligase activity remain unresolved. Our study adds to the understanding of E3-dependent functions of Cbl and Cbl substrates, which aids in elucidating its role under different situations.
Our study identifies a new role for Cbl and a novel PI3K kinase activity–independent function of p85, both in promoting EpoR endocytosis and downregulation. This process critically controls EpoR signaling and, when defective, contributes to red blood cell hyperproliferation in PFCP. Because there is an increasing association of ubiquitin-mediated pathways in pathological conditions, the identification and functional characterization of E3 ligases and their contribution to diseases are important in uncovering their function under physiological and pathological conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Yue Ma for technical assistance and Drs Nai-Wen Chi, Peter Michaely, and Joachim Seemann for helpful discussions. The authors also thank Dr Wallace Langdon, Dr Brian Druker, and Dr Harvey Lodish.
This study was supported by research funding from the National Institutes of Health (R01 HL089966) (L.J.H.) and a predoctoral fellowship from the American Heart Association (12PRE12050317) (G.B.B.). This work was performed in laboratories constructed with support from National Institutes of Health grant C06 RR30414.
Authorship
Contribution: G.B.B., R.S., H.Y., and L.J.H. designed and performed research and analyzed data; and G.B.B. and L.J.H. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lily Huang, PhD, Assistant Professor, Department of Cell Biology, University of Texas Southwestern Medical Center, 6000 Harry Hines Blvd. NL6.138A, Dallas, TX 75390-9039; e-mail: lily.huang@utsouthwestern.edu.