Abstract
Choosing Wisely® is a medical stewardship and quality improvement initiative led by the American Board of Internal Medicine Foundation in collaboration with leading medical societies in the United States. The ASH is an active participant in the Choosing Wisely® project. Using an iterative process and an evidence-based method, ASH has identified 5 tests and treatments that in some circumstances are not well supported by evidence and which in certain cases involve a risk of adverse events and financial costs with low likelihood of benefit. The ASH Choosing Wisely® recommendations focus on avoiding liberal RBC transfusion, avoiding thrombophilia testing in adults in the setting of transient major thrombosis risk factors, avoiding inferior vena cava filter usage except in specified circumstances, avoiding the use of plasma or prothrombin complex concentrate in the nonemergent reversal of vitamin K antagonists, and limiting routine computed tomography surveillance after curative-intent treatment of non-Hodgkin lymphoma. We recommend that clinicians carefully consider anticipated benefits of the identified tests and treatments before performing them.
Introduction
Choosing Wisely® is a medical stewardship campaign spearheaded by the American Board of Internal Medicine (ABIM) Foundation in collaboration with leading American medical professional societies and consumer groups. The ABIM Foundation is a nonprofit organization established by the ABIM that aims to foster medical professionalism and quality improvement. The Choosing Wisely® campaign challenges medical societies to identify 5 tests, procedures, or treatments within each specialty's clinical domain that are offered to patients despite an absence of evidence demonstrating benefit or, in some cases, despite evidence demonstrating disutility or harm.
The Choosing Wisely® campaign aims to encourage dialogue among patients, physicians, and the community at large about the cost and benefits of medical care. The need for this dialogue is highlighted by the observation that at least 27% of investigations ordered on admission are avoidable, increasing to 63% on subsequent days.1 The Institute of Medicine estimates that 1 of 3 dollars spent on healthcare is wasted and that diagnostic testing is particularly inefficient.2 Furthermore, a growing body of literature suggests that reducing unneeded investigations cannot only decrease costs, but can also in many cases increase patient satisfaction and quality of care.2,3
The ASH is an active participant in the Choosing Wisely® project and, through a rigorous, evidence-based methodology outlined below, has identified 5 tests and treatments that practicing hematologists should carefully consider because, in the circumstances described, the risk of harm and/or cost of the specified interventions likely outweigh the anticipated benefits.
Methods
In August 2012, the ASH Choosing Wisely® Task Force (CWTF) was formed and asked to identify 5 hematologic tests, procedures, or treatments that physicians and/or patients should question. The ASH CWTF included 11 members with expertise in adult, pediatric, malignant, benign, and laboratory hematology.
The ASH Choosing Wisely® item selection process was anchored by 5 core principles (Table 1). Four of these principles (numbers 2-5) were recommended by the ABIM Foundation. ASH chose to explicitly identify harm to patients as a fifth and preeminent guiding principle for our selection process. Therefore, tests, procedures, or treatments that involved greater risk of harm to patients and had limited evidence of utility were prioritized over tests, procedures, or treatments that had limited evidence of utility, but lower risk of harm.
Suggestions for Choosing Wisely® items were solicited from the membership of 8 practice-, quality-, education-, and evidence-oriented ASH committees, as well as from members of the ASH Practice Partnership and the ASH “Consult-a-Colleague” service. Subscribers to the ASH Practice Update were also invited to make submissions.
Using nominal group technique,4 the ASH CWTF reduced the list of suggested Choosing Wisely® items to a short list of 20. Members of the ASH committees outlined above were asked to score each item on this short list from 1 to 10 with regard to priority for inclusion in ASH's final Choosing Wisely® list. The ASH CWTF used these scores and the guiding principles in Table 1 to reduce the list to 10 items.
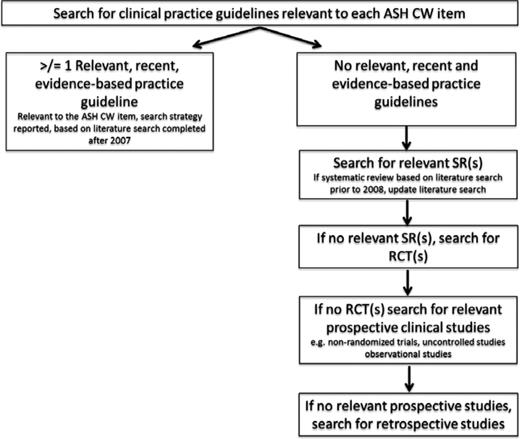
A systematic review of the evidence was completed for each of the 10 items on the final short list. As illustrated in Figure 1, a hierarchical search strategy was used such that the search was abridged if recent (published subsequent to 2008), relevant, evidence-based guidelines were identified (see Appendix 1A for search terms). The search was restricted to English language publications indexed in Medline (2008-2012 for guidelines; 1946 through December 2012 for primary literature), the National Guideline Clearinghouse, and the Canadian Medical Association Infobase. The Cochrane Database of Systematic Reviews (CDSR; Issue 12, 2012) was included in searches for systematic reviews. Searches of the websites of the Scottish Intercollegiate Guidelines Network (SIGN) and the British Committee for Standards in Haematology (BCSH) were also conducted (http://www.sign.ac.uk and http://www.bcshguidelines.com, respectively; accessed January 14, 2013). One reviewer (A.H.) performed an initial review of titles and abstracts. Two reviewers (L.H. and A.H.) reviewed the full text of potentially eligible citations. Reference lists of included guidelines or studies were also reviewed for potentially relevant studies. Disagreements regarding eligibility were resolved through consensus.
Hierarchical search strategy used in the systematic reviews completed to support the ASH Choosing Wisely® project. CW indicates Choosing Wisely®; SR, systematic review; and RCT, randomized controlled trial.
Hierarchical search strategy used in the systematic reviews completed to support the ASH Choosing Wisely® project. CW indicates Choosing Wisely®; SR, systematic review; and RCT, randomized controlled trial.
An evidence summary was prepared for each item. The ASH CWTF reviewed the evidence summaries and the supporting documents for the 10 items on the final short list. Based on the evidence, the ASH CWTF elected to make a minor modification to one item on the short list and a substantial modification to a second item. To ensure that the evidentiary base was accurate, the literature searches for these 2 items were redone using updated search strategies that reflected the changes made to the items. Using nominal group technique4 informed by the evidence summaries and guided by the principles in Table 1, the ASH CWTF selected 5 final items plus 1 alternate for the ASH Choosing Wisely® Campaign. The final items were each reviewed by 2 or more external content experts for accuracy and clarity.
Results
One-hundred and sixty-seven ASH committee members were solicited for suggestions; 57 (35%) provided one or more suggested tests, procedures, or treatments for physicians and their patients to question. Two subscribers to the ASH Practice Update also provided suggestions. A total of 154 suggestions were received, representing 81 unique items. Four items overlapped substantially with recommendations from other professional societies in previous Choosing Wisely® Campaigns; the ASH CWTF elected to exclude 3 of these items (Table 2). One overlap item was retained due to a slightly different focus than its predecessor and because the ASH CWTF felt that this item (regarding RBC transfusion) was central to hematology practice. The initial short list of 10 items is listed in Table 4 in Appendix 1B.
In May 2013, 5 ASH Choosing Wisely® items and one alternate were submitted to the ABIM Foundation. Minor language changes were recommended by the ABIM Foundation and were endorsed by the ASH CWTF. The ABIM Foundation also recommended that one item involving diagnostic testing for lymphoma be removed in favor of a recommendation regarding surveillance computed tomography (CT) scans in aggressive non-Hodgkin lymphoma (NHL) due to a concern that the diagnostic recommendation focused more on appropriateness of testing than on overuse of testing. The final ASH CW items, including the deferred item, are listed in Table 3.
Table 3 summarizes the key references supporting each of the ASH Choosing Wisely® recommendations. Four of the 5 final recommendations were supported by recently published, evidence-based guidelines. One item (item #5) was supported by guidelines that were not clearly evidence based,5,6 so for this item, our systematic review included the primary literature.
Discussion
The Choosing Wisely® campaign aims to encourage physicians and patients to question tests, procedures, or treatments that have limited evidence of utility in certain circumstances and that in aggregate contribute to the high cost of medical care. These aims are particularly salient to the field of hematology because hematology is a laboratory-based specialty dependent on a wide array of blood- and tissue-based tests and because the cost of contemporary hematology/oncology treatments is rapidly escalating.7-9
ASH has identified 5 hematologic tests and treatments that should be questioned in the circumstances indicated (Table 3). These 5 items were selected using a rigorous and reproducible methodology that sought input from an array of ASH committee members and incorporated evidence-based systematic reviews into the selection process. The methodology we developed could be adapted to future Choosing Wisely® campaigns or similar initiatives.
ASH's first recommendation advises against liberal transfusion of RBCs. Transfusion of the smallest effective dose of RBCs is recommended because, compared with restrictive strategies, liberal transfusion does not improve patient outcomes.10-14 Therefore, liberal transfusion generates costs and exposes patients to potential harms from transfusion without likelihood of benefit. Consistent with this recommendation, we further advise that clinicians avoid administering 2 units of RBCs if 1 unit is sufficient and that appropriate weight-based dosing of RBCs be used in children.
ASH's second recommendation advises against thrombophilia testing in adult patients diagnosed with venous thromboembolism (VTE) in the context of a major transient VTE risk factor such as surgery, trauma, or prolonged immobility.15,16 In this scenario, thrombophilia testing does not influence duration or intensity of treatment.17-19 In addition, thrombophilia testing has the potential to cause harm if the duration of anticoagulation is inappropriately prolonged or if patients are incorrectly labeled as having a thrombophilic disorder (which could influence subsequent insurability). One caveat to the above recommendation involves patients who experience VTE in the setting of a major transient risk factor but who have additional risk factors such as a positive family history or concurrent exposure to hormonal therapy. ASH recommends that such patients seek guidance from an expert in VTE.
ASH's third recommendation advises against the routine use of IVC filters. There is a paucity of evidence supporting the use of IVC filters.18,20 Existing guidelines agree that the main indication for an IVC filter is acute VTE plus a contraindication to anticoagulation.17,21-23 Possible indications for IVC filters include pulmonary embolism (PE) despite appropriate therapeutic anticoagulation and massive PE with poor cardiopulmonary reserve. Filters placed for primary prophylaxis of PE in patients who do not have acute deep vein thrombosis of the leg are widely used24 ; however, there is no evidence to support their utility and there is clear evidence that such filters cause harm.20 For example, in a recent report of 6376 patients undergoing bariatric surgery, prophylactic IVC filters did not reduce postoperative VTE, but did appear to increase the risk of death and/or disability.25
When IVC filters are necessary, retrievable filters are strongly recommended over permanent filters. ASH recommends that retrievable filters be removed as soon as the risk for PE has resolved and/or when anticoagulation can be safely resumed. Recent reports suggest that a minority of “temporary” filters are ever retrieved (between 8.5% and 34%).20,26 Therefore, clinicians are advised to consider developing a concrete plan for IVC removal at the time of IVC placement.
ASH's fourth recommendation advises against the use of plasma or prothrombin complex concentrates to reverse vitamin K antagonists (VKAs) in the absence of bleeding, emergent surgery, or emergent invasive procedures. The use of plasma or prothrombin complex concentrates to nonemergently reverse VKAs increases costs and exposes patients to potential harm from transfusion with little likelihood of benefit. Published evidence-based guidelines provide guidance on the optimal approach to the reversal of VKAs.27,28 Most nonbleeding patients can be managed by reducing or withholding VKAs or by administering small doses of vitamin K depending on the International Normalized Ratio (INR) and the clinical scenario. For nonbleeding patients with an INR greater than 10, there are no randomized controlled trials to guide practice. A small prospective cohort study suggests that most of these patients can be safely managed by administering small doses of vitamin K rather than with the use of blood products.29 In addition, it has been reported that at higher INR values, the linear relationship between vitamin K–dependent factor levels and the INR is lost and the INR becomes a less meaningful measure of bleeding risk.30 Given these limitations, we recommend that for patients with an INR greater than 10, physicians use predominantly clinical factors (such as bleeding and risk factors for bleeding) to determine the urgency with which VKA reversal should proceed.
ASH's fifth and final recommendation advises clinicians to limit the use of surveillance CT scans in asymptomatic patients in complete remission from aggressive NHL. In addition to their cost, CT scans deliver modest doses of radiation to patients and are associated with a small increased risk of malignancy over the long term.31 Our systematic review of the literature revealed no published evidence of a survival benefit from surveillance CT scans of asymptomatic aggressive NHL survivors.31-33 On the contrary, published reports suggest that most relapses are heralded by clinical symptoms.33-36 Even when relapses were detected earlier on a routine scans, there was no evidence of a survival benefit with more liberal surveillance strategies.37 In addition, a growing body of literature suggests that CT scans are associated with a measurable lifetime risk of secondary malignancy.38 Indeed, in some patient groups (such as young women with highly curable lymphoma), the estimated lifetime risk of cancer mortality associated with 10 CT scans approaches the 5-year cumulative probability of lymphoma death.31 Therefore, judicious use of CT surveillance is especially important in young patients with good-prognosis lymphoma.
Conclusion
In summary, the ASH Choosing Wisely® campaign has identified 5 tests and treatments that increase the cost of medical care and expose patients to potential risks with a low likelihood of benefit when used in the incorrect setting. In some cases, such as the recommendation against liberal transfusion of RBCs, there is a strong evidentiary basis for the recommendation. In other cases, such as the recommendations around IVC filter use and limiting CT surveillance of aggressive lymphoma, the recommendations are based largely on an absence of data supporting a practice in the face of potential harms and cost. In all cases, the recommendations are bounded by the current state of the science. As the evidence evolves, it is possible that certain recommendations will need to be revisited.
Ultimately, the real challenge for all of the Choosing Wisely® campaigns will be to determine whether they contributed to positive change in the actual delivery of patient care. Although clearly outside of the scope of the present article, efforts are under way to develop quality metrics and toolkits based on Choosing Wisely® items. If Choosing Wisely® is successful, it may be possible in some instances to demonstrate changes in practice through time trends in large, population-based datasets. In other cases, the main positive outcome of Choosing Wisely® may be to stimulate research in areas singled out by Choosing Wisely® as lacking a sufficient evidentiary basis. For the time being, we encourage physicians to consider the ASH Choosing Wisely® recommendations when they are providing clinical care, when they are teaching medical trainees, and when they are planning future research endeavors.
This article was selected by the Blood and Hematology 2013 American Society of Hematology Education Program editors for concurrent submission to Blood and Hematology 2013. It is reprinted in Hematology Am Soc Hematol Educ Program. 2013;2013:9-14.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by ASH. Suzanne Leous (ASH staff) provided administrative and organizational assistance to the project. Adam Haynes is a professional methodologist contracted by ASH. The following individuals acted as expert, external reviewers for one or more ASH Choosing Wisely® items: Jeannie Callum, Jeffrey Carson, Joe Connors, Mary Cushman, Mark Crowther, David Garcia, John Heit, Robert Weinstein, and Jane Winter.
Authorship
Contribution: L.K.H., H.B., K.R.C., J.K., V.K., A.M., B.U.M., S.H.O, M.P., R.S., and L.S. contributed to study design and implementation; L.K.H. and A.E.H. completed the systematic reviews; L.K.H. wrote the first draft of the manuscript; M.A.C. wrote sections of the manuscript; and all authors contributed to review and revisions of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests. Off-label drug use: None disclosed.
Correspondence: Lisa K. Hicks, 30 Bond St, Rm 2-084 Donnelly Wing, Toronto, ON, M5B 1W8, Canada; e-mail: hicksl@smh.ca.
Appendix
ASH Choosing Wisely® Task Force membership: L. K. Hicks (Chair), H. Bering, K. R. Carson, J. Kleinerman, V. Kukreti, A. Ma, B. U. Mueller, S. H. O'Brien, M. Pasquini, R. Sarode, and L. Solberg.