Abstract
One obstacle that continues to pose a major risk for hematopoietic stem cell transplantation is the occurrence of chronic graft-versus-host disease (cGVHD). Although the exact cause of cGVHD is unknown, preclinical findings have demonstrated a significant increase in class-switched anti-host reactive antibody deposition and fibrosis in target organs. We have previously demonstrated the dependence of cGVHD on donor bone marrow (BM)-derived B cells and splenic-derived T cells in a model of cGVHD that develops bronchiolitis obliterans (BO), a pathognomonic symptom of lung cGVHD (Blood 119:1570-80, 2012). T:B cell pathways responsible for cGVHD have yet to be elucidated. Germinal Center (GC) reactions are dependent on T follicular helper (TFH) cell stimulation and controlled by T follicular regulatory cells (TFR). TFH cells are located in the B-cell zones, express Bcl6, CXCR5, ICOS, and high levels of PD1, and provide stimulation through IL-21 production and ICOS and CD40L expression. TFR are located in the GC and express FoxP3. In this study we define a previously unknown role for TFH cells in the development of cGVHD.
B10.BR (H2k) mice were conditioned with cyclophosphamide and irradiation and transplanted with B6 (H2b) BM and mature splenic-derived T cells. After 8 weeks, pulmonary function of the mice was measured during mechanical ventilation using whole body plethymography. BO was defined by low compliance and high elastance and resistance in association with collagen deposition leading to obliterated bronchioles. Target organs (lung, liver, spleen) were analyzed for collagen accumulation, immunoglobulin deposition, size of GCs, frequency and of TFH, GC B cells and TFR. Interventions included use of knockout (KO) donors or administration of monoclonal antibody (mAb) from days 28-56 to treat cGVHD.
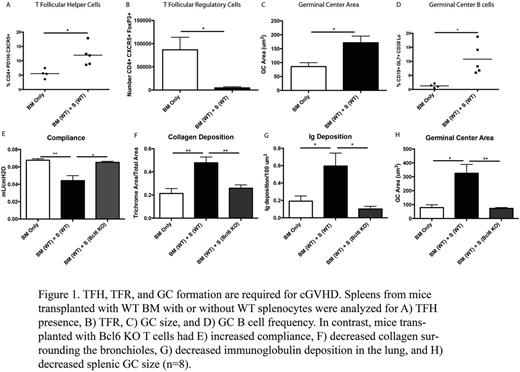
cGVHD is associated with high GC number, TFH cell number and low TFR (Figure 1 A-D). To determine whether TFH cells were required for cGVHD, we transplanted mice with wild-type (WT) BM and WT T cells or T cells deficient for the TFH master regulator Bcl-6. In contrast to WT T cells, recipients of BCL6 KO T cells had no pathogenic antibody deposited in the lung and no evidence of BO (Figure 1 E-H). Recipients of TFH deficient T cells had a significant decrease in the collagen surrounding the bronchioles and in frequency of GC B cells and TFH compared to cGVHD mice. Consistent with an essential role for TFH cells, TFH deficient, CXCR5 KO donor T cells were unable to induce cGVHD. To determine whether the TFH-produced cytokine IL-21 was necessary for cGVHD, donor IL-21 KO T cells with WT BM or WT T cells with IL-21R KO BM-derived B cells did not develop cGVHD. Importantly, therapeutic administration of anti-IL-21 neutralizing mAb (kindly provided by Novo Nordisk, Copenhagen, Denmark) was able to reverse established cGVHD in this model. Studies using TFH-deficient, ICOS KO donor T cells or anti-ICOS blocking mAb beginning day 28 as cGVHD therapy confirmed the requirement for ICOS:ICOS ligand signaling in cGVHD generation and maintenance. Moreover, blocking CD40L:CD40 interactions in mice with established cGVHD using anti-CD40L blocking mAb was effective in reversing cGVHD. Lastly, studies using a highly B cell depleting anti-CD20 mAb for cGVHD therapy suggested that plasmablasts and plasma cells derived from GC B cells were critical for continued pathogenic Ab production as cGVHD was not reversed by anti-CD20 mAb in this model system. However, it is unknown if prophylactic treatment with anti-CD20 mAb is effective for preventing onset of disease.
These data provide novel insights into cGVHD pathogenesis, indicating the essential role of TFH in these processes and suggest new lines of therapy using mAb that target TFH cells, GC B cells, and their interactions, with the potential to reverse established pulmonary cGVHD.
Browning:Biogen Idec: Employment.
Author notes
Asterisk with author names denotes non-ASH members.