Abstract
NOX-A12 is a novel, potent, L-stereoisomer RNA aptamer (Spiegelmer®) that binds and neutralizes CXCL12/SDF-1, a chemokine which attracts and activates immune- and non-immune cells via interaction with its receptors, CXCR4 and CXCR7. CXCL12 plays an important role in homing and trafficking of multiple myeloma (MM) cells to the bone marrow (BM). Here, we evaluate the capacity of NOX-A12 to mobilize plasma cells into the circulation. Furthermore, we analyze a possible therapeutic impact of NOX-A12 which disrupts the interaction of MM cells with the BM stroma resulting in enhanced chemosensitivity to established therapies such as bortezomib and dexamethasone (VD).
To date, 21/28 planned patients have been enrolled into a multicenter Phase IIa study of NOX-A12 alone and in combination with VD in relapsed MM patients. Here we report interim data on PK, PD and preliminary efficacy of a pilot group consisting of 3 cohorts of 3 patients each. In the pilot phase, cohorts received single doses of 1, 2 or 4 mg/kg NOX-A12 alone, respectively, two weeks prior to 8 planned cycles of combined treatment with NOX-A12 and VD repeated every 21 days. During combination therapy, NOX-A12 was administered 1-2 hours prior to bortezomib following a dose titration design for all patients: NOX-A12 doses were increased from 1 mg/kg to 2 mg/kg and 4mg/kg at cycles 1, 2 and 3, respectively. During cycles 4-6, doses of NOX-A12 were kept at the highest individually titrated dose. Bortezomib (1.3 mg/m2) was given on days 1, 4, 8 and 11 as intravenous injection. Oral dexamethasone (20 mg) was added on the day of and the day after bortezomib administration. Response was evaluated by applying the uniform response criteria of the IMWG 2011.
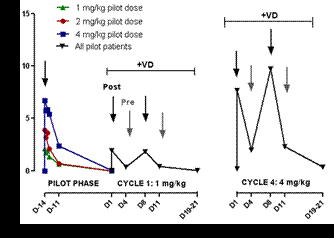
In total, 10 patients were enrolled into the pilot group (one patient was replaced after withdrawal of consent). The median age was 63 years (range 56-78) with 7 women and 3 men being included. Median prior therapies were 2 (range 1-5), with 9 patients having been pretreated with dexamethasone and 3 with bortezomib prior to enrollment. At screening 1, 7 and 2 patients presented with ISS stage I, II and III, respectively. IgG, IgA and LC M-components were present in 6, 3 and 1 patients, respectively. Six patients had cytogenetic aberrations, which were high risk (t(4;14) or del17p) in 2 patients. Plasma profiles of NOX-A12 in the patient population of the pilot group (Figure 1) were similar to healthy volunteers in which a plasma half-life of approximately 38 hours was observed. After single doses of NOX-A12, a dose-linear exposure with peak plasma levels of 2.1, 3.9, and 6.7 µM was found in the corresponding cohorts. Furthermore, an increase of plasma cells in peripheral blood by approximately 200% was detected which lasted throughout the observation period of 3 days (Figure 2). NOX-A12 as single agent was safe and very well tolerated.
Of nine evaluable patients who received the combination treatment of NOX-A12 and VD, 2 (22%) achieved a VGPR and 4 (44%) PR, resulting in an ORR (≥ PR) of 67%. In combination with VD, NOX-A12 was equally safe and well tolerated.
Kinetics of plasma levels in the cohorts of patients treated with different NOX-A12 doses (pilot phase) and in all patients together after combined treatment with NOX-A12 and VD (cycle 1 and 4)
Kinetics of plasma levels in the cohorts of patients treated with different NOX-A12 doses (pilot phase) and in all patients together after combined treatment with NOX-A12 and VD (cycle 1 and 4)
Mobilization kinetics of plasma cells after a single dose of NOX-A12 alone (n = 10).
Mobilization kinetics of plasma cells after a single dose of NOX-A12 alone (n = 10).
Proof of principle was achieved as single doses of NOX-A12 reached the expected plasma exposure and translated into an effective and prolonged mobilization of plasma cells into the peripheral blood. In addition, responses (PR or better) were noted in 6/9 (67%) patients of this pilot group. Single agent NOX-A12 was not associated with any relevant toxicity and the combination of VD with NOX-A12 was well tolerated and not associated with additional toxicity on top of VD. Provided that these promising findings will be confirmed in the total sample of 28 patients, further development of this novel anti-CXCL12/SDF-1 Spiegelmer® seems warranted.
Ludwig:Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Munipharma: Honoraria, Research Funding. Weisel:Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Engelhardt:MSD, Janssen-Cilag: Research Funding. Greil:NOXXON Pharma AG: Research Funding. Dümmler:NOXXON Pharma AG: Employment. Zöllner:NOXXON Pharma AG: Employment. Zeitler:NOXXON Pharma AG: Employment. Riecke:NOXXON Pharma AG: Employment. Kruschinski:Noxxon: Employment.
Author notes
Asterisk with author names denotes non-ASH members.