Abstract
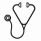
Although its prevalence is not well defined, transformation to Hodgkin lymphoma (HL) is a rare but well recognized complication in patients with CLL. Prior studies have reported a median survival of 0.8-1.7 years in these patients. Prior therapy of CLL, especially with purine analogs, has been shown to have adverse impact on survival after development of HL. We used the Mayo Clinic CLL database to describe outcomes of CLL patients seen at our institution who developed HL.
The Mayo Clinic CLL database includes all consenting patients with a pathologic diagnosis of CLL. Patients who developed biopsy-proven HL were included in this analysis. For patients referred from an outside institution, pathology review at Mayo Clinic was mandatory to be included. Clinical characteristics, therapy and overall survival (OS) were analyzed for all HL patients.
Among 3124 CLL patients seen at Mayo Clinic from 1/1995 to 7/2011, 26 developed HL. In a nested cohort of 1644 patients who were seen within 12 months of CLL diagnosis and followed prospectively, the prevalence of HL was 0.05%/year (10 year risk=0.5%). All 26 patients had classical HL. The median duration between CLL diagnosis and development of HL was 6.2 years (range, 0-24.5 years). The median age at diagnosis of HL was 68 years (range, 45-88 years) and 21 (81%) were male. At the time of HL diagnosis, the median WBC count was 7.8 x 109/L (range, 1.3-87.3), median hemoglobin was 11 gm/dL (range, 7.2-13), median platelet count was 220x109/L (range, 53-627), median serum lactate dehydrogenase was 249 U/L (range, 133-658), and median serum albumin was 3.4 gm/dL (range, 2.6-4.2). According to the Ann Arbor staging system for HL, 10 (38%) were Stage II, 2 (8%) were Stage III, 11 (42%) were Stage IV, and undetermined in 3 (12%) patients. Sixteen patients (61%) received HL therapy with doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD), seven (27%) received other chemotherapy, and treatment was unknown in 3 (12%) patients. No patient underwent stem cell transplantation as therapy for HL.
After a median follow-up of 8.7 years from CLL diagnosis date, 16 (61%) patients have died. The median OS from date of HL diagnosis was 3.9 years (95% CI, 1.0 – 5.0 years). The International Prognostic score (IPS) for HL, originally proposed by Hasenclever in 1998, identified seven prognostic factors: serum albumin <4 gm/dL, hemoglobin <10.5 gm/dL, male sex, age >45 years, stage IV disease, WBC≥15 x 109/L, and lymphocytopenia in patients with newly diagnosed HL. When we applied these prognostic parameters (available in 22/26 patients, 85% in our cohort), the IPS score predicted median survival as follows: score of ≤2 (18% of the patients), median survival not reached; score of 3 (23% of the patients), 6.2 years; score of 4 (36% of the patients), 2.4 years; and score ≥5 (23% of the patients), 0.3 years (p-value=0.005, Figure 1). In contrast, the Richter syndrome prognostic score developed for CLL patients who transform to diffuse large B-cell lymphoma failed to predict survival in these patients (Tsimberidou, JCO, 2006).
Of the 26 patients with HL, 16 (61%) had received therapy for their CLL prior to developing HL, including 12 (46%) who had received more than one line of therapy. Purine analogs had been administered for treatment of CLL prior to development of HL in 13/26 (50%) patients. The median survival of HL patients who had previously received CLL therapy was significantly worse compared to those who were untreated for CLL (2.8 years vs. 7.6 years, p-value=0.02). There was a trend toward shorter survival for HL patients who had received previous purine analog therapy for CLL compared to those who received alternate therapies (1.8 years vs. 5 years, p-value: p-value=0.07).
Of the 9 HL biopsy samples in which Epstein Barr virus (EBV) in-situ hybridization studies were performed, EBV was detected in 6 (67%) patients. No association was observed between prior purine analog therapy and EBV status (p-value=1.0). There was no difference in survival according to the EBV status of HL (p-value=0.33).
The 10 year incidence of transformation to Hodgkin transformation among patients with newly diagnosed CLL is 0.5% (∼1/200 CLL patients). In this single-institution analysis, median survival of CLL patients who developed Hodgkin transformation of CLL was approximately 4 years after HL diagnosis. The IPS accurately predicted survival in our cohort of HL patients.
Zent:Novartis: Research Funding; GlaxSmithKline: Research Funding; Biothera: Research Funding; Genzyme: Research Funding; Genentech : Research Funding. Shanafelt:Glaxo-Smith-Kline: Research Funding; Cephalon: Research Funding; Hospira: Research Funding; Celgene: Research Funding; Polyphenon E International: Research Funding; Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract