Abstract

Although the clinical importance of del 17p13 in patients with chronic lymphocytic leukemia (CLL) is well recognized, the implications of when this defect is identified are less clearly defined. In particular, the distinction between the identification of del 17p13 at the time of diagnosis and secondary acquisition of del 17p13 during the course of the disease are poorly characterized.
We identified all patients with CLL cared for at the Mayo Clinic between 1/1/2000 and 12/31/2012 who underwent baseline fluorescence in-situ hybridization (FISH) testing prior to receiving treatment. The Results of repeat FISH testing were reviewed to identify cases with clinically ascertained clonal evolution.
A total of 1757 patients with newly diagnosed CLL were seen at Mayo Clinic prior to receiving treatment. Among these, 1243 had FISH testing performed prior to treatment and within 36 months of diagnosis. The median time from diagnosis to initial FISH was 0.8 months (11.5 to 35.4 months). The Results of baseline FISH testing among these patients is as follows: 486 (39%) had del 13q14, 234 (19%) had trisomy 12, 115 (9%) had del 11q23, 59 (5%) had del 17p13, 15 (1%) had other (e.g., del6q) and 334 (37%) had no abnormalities detected.
Among these patients, 344 (28%) underwent repeat FISH testing during the course of their disease. Repeat FISH testing was typically performed for clinical suspicion of karyotype evolution or prior to therapy selection in patients with a long time interval between first prognostic FISH and initiation of treatment. Among these 344 patients, 41 (12%) acquired new cytogenetic abnormalities on repeat FISH. Classification of these 344 patients by the Dohner classification at diagnosis and time of follow-up FISH is shown in Table 1. Among the 41 pts who acquired a new FISH detectable genetic abnormality, the newly acquired defect resulted in a change in Dohner FISH risk category for 39 patients including 15/41 (37%) who acquired a del 17p13. Baseline clinical and prognostic characteristics of patients who developed clonal evolution to those who did not are shown in Table 2. Patients with unmutated immunoglobulin heavy chain (IGHV) gene mutation status (p<0.0001) and CD49d (p=0.003) expression were more likely to experience clonal evolution.
| . | Subsequent FISH . | ||||||
|---|---|---|---|---|---|---|---|
| First FISH . | Normal . | Other . | del13q . | +12 . | del11q . | del17p . | Change in Dohner category . |
| Normal | 51 | 1 | 8 | 6 | 3 | 4 | 7 |
| Other | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| del13q14 | 6 | 0 | 110 | 1 | 0 | 3 | 4 |
| +12 | 8 | 0 | 1 | 63 | 4 | 7 | 11 |
| del11q23 | 4 | 0 | 0 | 1 | 34 | 1 | 1 |
| del17p13 | 5 | 0 | 0 | 0 | 0 | 20 | 0 |
| . | Subsequent FISH . | ||||||
|---|---|---|---|---|---|---|---|
| First FISH . | Normal . | Other . | del13q . | +12 . | del11q . | del17p . | Change in Dohner category . |
| Normal | 51 | 1 | 8 | 6 | 3 | 4 | 7 |
| Other | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| del13q14 | 6 | 0 | 110 | 1 | 0 | 3 | 4 |
| +12 | 8 | 0 | 1 | 63 | 4 | 7 | 11 |
| del11q23 | 4 | 0 | 0 | 1 | 34 | 1 | 1 |
| del17p13 | 5 | 0 | 0 | 0 | 0 | 20 | 0 |
| Characteristic . | Patients with clonal evolution, N=39, (%) or [range] . | Patients without clonal evolution, N=305, (%) or [range] . | p-value . | |
|---|---|---|---|---|
| Median Age, years (range) | 58 (39-85) | 61 (25-87) | 0.13 | |
| Male | 27 (69%) | 225 (74%) | 0.55 | |
| Rai Stage | Low risk (0) | 12 (31%) | 147 (48%) | 0.12 |
| Intermediate risk (I-II) | 24 (62%) | 138 (45%) | ||
| High risk (III-IV) | 3 (8%) | 20 (7%) | ||
| IGHV mutation* | Mutated | 4 (11%) | 131 (48%) | <0.0001 |
| Unmutated | 31 (89%) | 141 (52%) | ||
| ZAP-70* | Negative | 18 (53%) | 158 (58%) | 0.62 |
| Positive | 16 (47%) | 117 (43%) | ||
| CD38* | Negative | 21 (55%) | 189 (63%) | 0.33 |
| Positive | 17 (45%) | 109 (37%) | ||
| CD49d* | Negative | 11 (33%) | 140 (61%) | 0.003 |
| Positive | 22 (67%) | 91 (39%) | ||
| Characteristic . | Patients with clonal evolution, N=39, (%) or [range] . | Patients without clonal evolution, N=305, (%) or [range] . | p-value . | |
|---|---|---|---|---|
| Median Age, years (range) | 58 (39-85) | 61 (25-87) | 0.13 | |
| Male | 27 (69%) | 225 (74%) | 0.55 | |
| Rai Stage | Low risk (0) | 12 (31%) | 147 (48%) | 0.12 |
| Intermediate risk (I-II) | 24 (62%) | 138 (45%) | ||
| High risk (III-IV) | 3 (8%) | 20 (7%) | ||
| IGHV mutation* | Mutated | 4 (11%) | 131 (48%) | <0.0001 |
| Unmutated | 31 (89%) | 141 (52%) | ||
| ZAP-70* | Negative | 18 (53%) | 158 (58%) | 0.62 |
| Positive | 16 (47%) | 117 (43%) | ||
| CD38* | Negative | 21 (55%) | 189 (63%) | 0.33 |
| Positive | 17 (45%) | 109 (37%) | ||
| CD49d* | Negative | 11 (33%) | 140 (61%) | 0.003 |
| Positive | 22 (67%) | 91 (39%) | ||
These parameters were not available for all patients
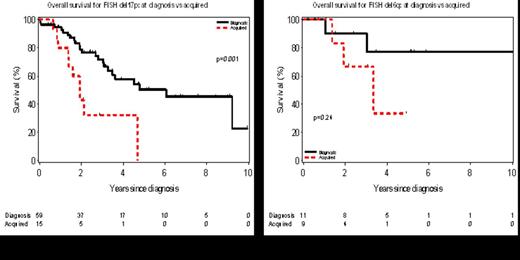
Among the 319 patients who did not have del 17p13 on baseline FISH, 15 (5%) acquired del 17p13 during the course of their disease. The overall survival from the date del 17p13 was identified is shown for those with del 17p13 at diagnosis (n=59) compared to those who acquired del 17p13 later in the course of the disease (n=15) in Figure 1A. Similarly, among 339 patients who did not have del 6q23 on baseline FISH, 9 (3%) acquired del 6q23 during the course of their disease. The overall survival from the date del 6q23 was identified is shown for those with del 6q23 at diagnosis (n=11) and those who acquired del 6q23 later in the course of the disease (n=9) in Figure 1B.
Acquired cytogenetic evolution is clinically ascertained by FISH during the course of the disease in approximately 12% of patients. The newly acquired defects in these patients result in a change in Dohner classification for in >90% of these patients including ∼37% who acquire del 17p13. The clinical implications of del 17p13 is influenced by the timing of ascertainment with markedly shorter survival for those who acquire del 17p13 during the course of the disease relative to those with this defect at diagnosis.
Shanafelt:Genentech: Research Funding; Glaxo-Smith-Kline: Research Funding; Cephalon: Research Funding; Hospira: Research Funding; Celgene: Research Funding; Polyphenon E International: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal