Abstract
Despite the initial response to combination chemotherapy in CLL/SLL patients, disease relapse and minimal residual disease (MRD) remain major issues in treatment of these diseases. Therefore, developing more effective treatments for CLL/SLL patients is a necessity. One strategy is to eliminate persistent disease using radioimmunotherapy (RIT), such as 131I-tositumomab (Bexxar®), as consolidation after an objective complete (CR) or partial response (PR) after an induction regimen. In this study we investigated the tolerability and efficacy of standard non-myeloablative doses of 131I-tositumomab following primary induction chemotherapy in CLL/SLL patients in first remission.
Patients older than 18 with CLL/SLL with indication for treatment were included if they were in first CR or PR from prior treatments and had <25% bone marrow involvement and acceptable peripheral counts. 131I-tositumomab (75 cGy total-body dose) was delivered between days 90 and 180 from the first day of the last chemotherapy treatment. The dose was reduced to 65 cGy in cases of thrombocytopenia (between 100,000-150,000/µL). Three months after the treatment dose, efficacy and response criteria were specified per NCI working group guidelines and toxicity assessments were recorded based on the CTCAEv3.0. Rituximab levels were determined using ELISA with a monoclonal anti-rituximab idiotype antibody.
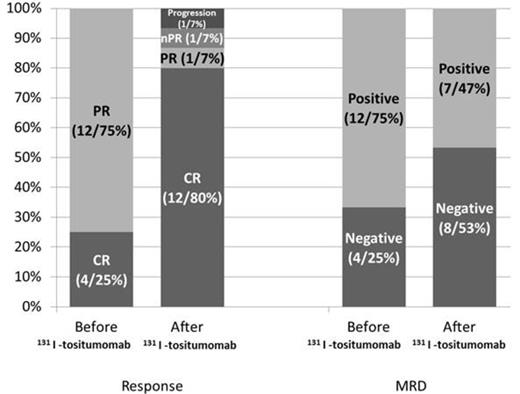
16 patients (CLL11, SLL 5) received consolidative 131I-tositumomab in first remission between 2005-2012. The median age was 61 (38-78). Seven patients (43.7%) had high-risk disease based on cytogenetics or molecular profile. Two patients (12.5%) had 11q deletion and one had mutated TP53 (6.25%). Increased CD38 and ZAP-70 expression was present in 3 of 11 and 6 of 7 patients who were tested and unmutated IgVH was detected in 1 of 3 patients. Twelve patients (75%) were in PR when entered the study while 4 (25%) were in CR. Eight patients (50%) had minimal residual disease (MRD) assessed by multiparametric flow cytometry (MFC). Prior chemotherapy consisted of FR in 9 patients (56.2%), FCR in 4 patients (25%), BR in 2 patients (12.5%) and R-CHOP in 1 patient (6.2%). The median time from the first day of the last treatment cycle to the RIT dose was 15.4 weeks (10 - 29). 7 patients (43.7%) needed dose reduction. At 3 months, CR was achieved or sustained in 12 patients (80%). Conversion from PR to CR following RIT occurred in 4 of 8 patients (50%). Likewise, 131I-tositumomab eliminated MRD in 4 of 8 patients (50%) by negative MFC at 3 months. One patient (6.6%) had PR, one (6.6%) had nodular PR and one had disease progression (6.6%) after administration of 131I-tositumomab. Lymphadenopathy was improved in 83% (5 of 6) of the patients with measureable disease prior to 131I-tositumomab. Overall, the patients with CR at 3 months had significantly higher levels of pre-treatment Rituximab levels (11.0 vs. 2.32 µg/ml, p = 0.01. There was no difference in the pre-treatment levels in patients with pre-treatment MRD who had a negative MFC analysis at 3 months compared to the ones to had residual MRD (12.5 vs. 7.6 µg/ml, p = 0.50) or in patients with PR before receiving 131I-tositumomab who achieved CR at 3 months compared to the ones who did not (11.7 vs. 5.8 µg/ml, p = 0.19). These Results suggest that the levels of Rituximab may be blocking CD20 sites at the lymph node and/or marrow level. The median follow-up was 9.4 months (2.7-54.3). Hematologic toxicities at 3 months were grade 3 anemia in 1 patient (6.2%), grade 3 or 4 neutropenia in 13 (81%), and grade 3 or 4 thrombocytopenia in 8 (50%). Six patients (37.5%) required blood or platelet transfusions. Two patients (12.5%) needed myeloid growth factor support. One patient was hospitalized within 3 months of RIT for neutropenia-related sepsis/typhlitis. Two patients (12.5%) had disease progression and dysplastic changes were found in one. One patient died 3 years after treatment for an unrelated medical reason. Persistent cytopenias were reported in 4 patients (25%) during the follow-up period.
Overall, consolidation RIT with 131I-tositumomab after first remission appears to be a feasible approach and may provide the potential benefit of converting PR to CR or eliminating MRD in CLL/SLL patients. Further long–term follow-up to assess possible prolonged side effects and clinical effectiveness of this approach remain on-going.
Off Label Use: Bexxar is not FDA approved for consolidation of CLL. Gopal:GSK : Research Funding. Becker:Pfizer: Consultancy. Maloney:GSK: Consultancy. Press:Roche/Genentech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal