Key Points
Fully human anti-hepcidin Abs have been generated for use as a potential therapeutic to treat AI.
The mechanism of action was shown to be due to an increase in available serum iron leading to enhanced red cell hemoglobinization.
Abstract
Iron maldistribution has been implicated in the etiology of many diseases including the anemia of inflammation (AI), atherosclerosis, diabetes, and neurodegenerative disorders. Iron metabolism is controlled by hepcidin, a 25-amino-acid peptide. Hepcidin is induced by inflammation and causes iron to be sequestered within cells of the reticuloendothelial system, suppressing erythropoiesis and blunting the activity of erythropoiesis stimulating agents (ESAs). For this reason, neutralization of hepcidin has been proposed as a therapeutic treatment of AI. The aim of the current work was to generate fully human anti-hepcidin antibodies (Abs) as a potential human therapeutic for the treatment of AI and other iron maldistribution disorders. An enzyme-linked immunosorbent assay was established using these Abs to identify patients likely to benefit from either ESAs or anti-hepcidin agents. Using human hepcidin knock-in mice, the mechanism of action of the Abs was shown to be due to an increase in available serum iron leading to enhanced red cell hemoglobinization. One of the Abs, 12B9m, was validated in a mouse model of AI and demonstrated to modulate serum iron in cynomolgus monkeys. The 12B9m Ab was deemed to be an appropriate candidate for use as a potential therapeutic to treat AI in patients with kidney disease or cancer.
Introduction
Hepcidin is the global regulator of iron metabolism.1 It is produced in the liver, secreted into serum, and binds to the iron export protein ferroportin, causing its internalization2 and subsequent degradation.3 Ferroportin controls iron release from gut enterocytes and also from macrophages of the reticuloendothelial system, which process iron from senescent red blood cells (RBCs) and intravenous iron administered to treat anemia.4,5 Although iron is needed for all metabolic processes, the largest iron demand is in developing erythrocytes where it is incorporated into hemoglobin (Hb) to transport oxygen.
Hepcidin is homeostatically regulated to maintain serum iron within defined limits6-8 but can also be induced by inflammation.9,10 Anemia is observed in several patient populations that exhibit inflammation, such as patients with severe trauma, chronic kidney disease (CKD), and cancer. Such anemia is often described as the anemia of inflammation (AI). Hepcidin is also cleared by the kidney11 and so is elevated in CKD patients even in the absence of overt inflammation.12
We have previously demonstrated that neutralizing hepcidin in a mouse model of AI effectively treated anemia in combination with erythropoiesis stimulating agent (ESA) treatment.13 In the present work, fully human anti-hepcidin antibodies (Abs) were generated and evaluated both as hepcidin detection reagents and as potential candidates for hepcidin neutralization in patients.
Materials and methods
Anti-hepcidin Ab generation
All mice were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Edition (supplemental Methods). Fully human anti-hepcidin Abs were generated using XenoMouse, IgG2κλ and IgG4 κλ, transgenic mice. Mice were injected 13 times over a period of 8 weeks using a combination of TiterMax Gold adjuvant (Sigma-Aldrich, Oakville, ON, Canada) and Alum adjuvant prepared from aluminum potassium sulfate (EMD Chemicals Inc., Gibbstown, NJ) and rhHepc conjugated to keyhole limpet hemocyanin (Thermo Scientific, Rockford, IL). Spleen and lymph tissues were harvested, and hybridomas were prepared using standard methods; 23 040 IgG2 hybridoma supernatants and 11 520 IgG4 hybridoma supernatants were screened (detailed screening methods are in the supplemental Methods). Binding screens identified 617 IgG2-specific and 1013 IgG4-specific binders. All IgG4 Abs selected for further characterization were immunoglobulin chain switched to IgG2. The binding affinities of these Abs to human hepcidin were determined by Biacore and confirmed by KinExA if KD < 400 pM. The KD values for the lead Abs were in the range of between 1 pM and 400 pM. Mouse anti-mouse hepcidin Ab 2C10 was generated in Hep2 mice (no mouse hepcidin present)13 similarly to the method described previously for anti-human hepcidin Abs.
Modified Brucella abortus AI mouse model
Heat-killed Brucella abortus (BA) was prepared, stored, and administered intraperitoneally to mice as detailed previously.13 Hep1 (mice with hHepc knocked into the mHepc1 locus13 ), Hep2-C57/BL/6 (mice with hHepc knocked into a locus spanning mHepc1 and 213 and so lacking all mouse hepcidin gene expression: fully backcrossed [Max-Bax speed congenics; CRL, Wilmington, MA]), or C57BL/6 mice were injected with BA (2-5 × 108 particles per mouse). Treatment with anti-hepcidin Abs, ESA, or saline control was conducted as specified in individual figure legends. In studies conducted to determine red cell response or serum iron response to anti-hepcidin Ab treatment, Hb was measured 6 to 7 days after BA treatment to determine which mice had developed anemia. Mice with an Hb value >95% confidence interval of the mean for all BA-treated animals were excluded from the study as detailed previously.13 Mice were then assigned to groups such that all groups had equivalent Hb values (matched mean ± standard deviation) prior to treatment. In studies designed to examine early modulation of BA-dependent cytokine production or endogenous erythropoietin response by Ab treatment, BA and Ab were administered concomitantly, and so no Hb-based exclusion criteria could be applied. For this reason, all mice injected with BA were analyzed. Blood cell parameters and serum iron were determined by a clinical laboratory analyzer as described previously.13 Serum cytokine concentrations were determined using a Luminex assay (Life Technologies). Endogenous erythropoietin concentrations were determined by Meso Scale Discovery assay (Meso Scale Discovery, Gaithersburg, MD).
Treatment with 12B9m in cynomolgus monkeys
For pharmacokinetic/pharmacodynamic (PK/PD) assessment of 12B9m, a single-dose intravenous injection of 50 mg/kg was administered to drug-naïve male cynomolgus monkeys. Blood samples were collected, and serum iron, 12B9m concentration, and total serum hepcidin were measured before Ab injection and at subsequent time points (30 minutes, 4 hours, 1 day, 2 days, 4 days, and 7 days). To assess PD response to repeated injections, both male and female drug-naïve cynomolgus monkeys were administered intravenous 12B9m at 5 mg/kg, 40 mg/kg, and 300 mg/kg weekly for 4 weeks. Blood samples were collected, and serum iron, 12B9m concentration, and total serum hepcidin were measured before Ab injection in each cycle (trough measurements). For the first and fourth cycles, samples were also collected after 6 hours, 1 day, 2 days, 4 days, and 7 days. Total cynomolgus serum hepcidin liquid chromatography–tandem mass spectrometry (LC-MS/MS) and total human Ab concentration were determined as previously described.14 Serum iron was measured as described for mouse.
Statistics
Statistics were generated using GraphPad Prism software v.5.04 (GraphPad Software, San Diego, CA). Individual tests used are detailed in the figure legends.
Results
Characterization of Ab-binding epitopes on hepcidin
As well as being a therapeutic target, hepcidin has been suggested as an informative biomarker for AI15 and other disorders.16 Mass spectroscopic assays17-20 and some immunoassays21 using polyclonal Abs have been shown to reliably detect hepcidin but have limits in terms of cost, ease of transfer to a hospital laboratory setting, or scalability and reproducibility for assays involving polyclonal Abs. For this reason, we and others have developed sandwich enzyme-linked immunosorbent assays (ELISAs) using monoclonal Abs to detect hepcidin.22,23
To determine if a monoclonal Ab sandwich ELISA against hepcidin was possible, experiments were conducted using polyclonal Abs raised against keyhole limpet hemocyanin–conjugated mature human hepcidin. Two rabbit anti-hHepc polyclonals were each tested for the ability to sandwich (Figure 1A; supplemental Methods). Both were capable of sandwiching, indicating that they contained Abs that recognized 2 or more epitopes on hepcidin simultaneously. The maximal signal achievable was <1 OD450, suggesting that sandwiching was a relatively rare event.
Characterization of hepcidin-binding Abs and development of a sandwich ELISA. (A) Polyclonal Abs against human hepcidin demonstrated that construction of a sandwich ELISA was possible. Each polyclonal Ab (4364 and 4366) was tested as both the capture Ab (eg, 4364 bound to ELISA plate) and detection Ab (4364 conjugated to horseradish peroxidase for detection). (B) The ability of 2 Abs to bind to hepcidin simultaneously was profiled by Biacore. Representative plot showing a panel of Abs, some of which bound to Ab 19D12 coated on a chip and treated with hepcidin. Binding of the second Ab was demonstrated by an increase in detected mass (increase in relative units [RU] of binding). (C) Diagram categorizing hepcidin-binding Abs into classes based on ability to recognize overlapping or distinct epitopes on hepcidin. Solid line indicates classes that could bind hepcidin simultaneously. (D) Comparison of performance of anti-human hepcidin sandwich ELISA with hepcidin detection by LC-MS/MS. Serum hepcidin concentrations from CKD patient samples were measured in both assays and compared (n = 46). Hepcidin ELISA (x-axis) and LC-MS/MS (y-axis).
Characterization of hepcidin-binding Abs and development of a sandwich ELISA. (A) Polyclonal Abs against human hepcidin demonstrated that construction of a sandwich ELISA was possible. Each polyclonal Ab (4364 and 4366) was tested as both the capture Ab (eg, 4364 bound to ELISA plate) and detection Ab (4364 conjugated to horseradish peroxidase for detection). (B) The ability of 2 Abs to bind to hepcidin simultaneously was profiled by Biacore. Representative plot showing a panel of Abs, some of which bound to Ab 19D12 coated on a chip and treated with hepcidin. Binding of the second Ab was demonstrated by an increase in detected mass (increase in relative units [RU] of binding). (C) Diagram categorizing hepcidin-binding Abs into classes based on ability to recognize overlapping or distinct epitopes on hepcidin. Solid line indicates classes that could bind hepcidin simultaneously. (D) Comparison of performance of anti-human hepcidin sandwich ELISA with hepcidin detection by LC-MS/MS. Serum hepcidin concentrations from CKD patient samples were measured in both assays and compared (n = 46). Hepcidin ELISA (x-axis) and LC-MS/MS (y-axis).
A range of anti-human hepcidin Abs were tested by Biacore analysis for their ability to bind to hepcidin simultaneously (Figure 1B). In the representative experiment shown, fully human anti-hHepc monoclonal Ab 19D12 (KD = 340 pM) was coated onto a chip, hepcidin was bound, and the ability of other Abs to bind additively was detected (supplemental Methods). Relative binding values are shown in Table 1. Abs generally fell into 3 categories (Figure 1C). The first class, embodied by Ab 19D12, represented the majority of the fully human Abs tested and was able to sandwich with the second class, embodied by 23F1124 (KD = 40 pM). This second class represented only ∼1% of the original pool of fully human anti-hHepc Abs, suggesting that recognition of this epitope was a relatively rare event. The third class of Abs, represented by mouse monoclonal Ab 2.7 (KD = 110 pM),13 was not capable of sandwiching, suggesting that this class was binding to a site overlapping the other 2 epitopes.
A sandwich ELISA was built using 19D12 for capture and 23F11 for detection (supplemental Methods). The resulting ELISA showed good concordance with the previously established LC-MS/MS assay19,22 (Figure 1D). The limit of sensitivity was 30 pg/mL, and a similar assay was established in Meso Scale Discovery format (19D12 and 15E1; data not shown).
Fully human anti-hHepc Ab 12B9m effectively treated ESA-refractory anemia in a modified BA model of AI
We previously demonstrated that a mouse anti-human Ab could neutralize hepcidin and restore response to ESA in a mouse model of AI.13 To examine the relevance of this observation for treating human disease, an Ab suitable for human administration was required.
From the panel of fully human Abs generated (1-400 pM), one of the highest affinity Abs (12B9m: KD = 2 pM; confidence interval 0.4-4 pM) was tested for the ability to neutralize hepcidin similarly to the proof-of-concept mouse anti-human hepcidin Ab 2.7. A modified version of the previously published BA AI model was used (schema detailed in Figure 2A). In the previous study, Hep1 or Hep2 mice were used (mice with hHepc in the mHepc1 locus: ortholog replacement; or in the mHepc1 and 2 locus: total homolog replacement). In the current study, mice with human hepcidin knocked in to the mouse Hepc1 and 2 locus,13 fully backcrossed onto C57BL/6, were used for the analysis (designated Hep2 C57BL/6).
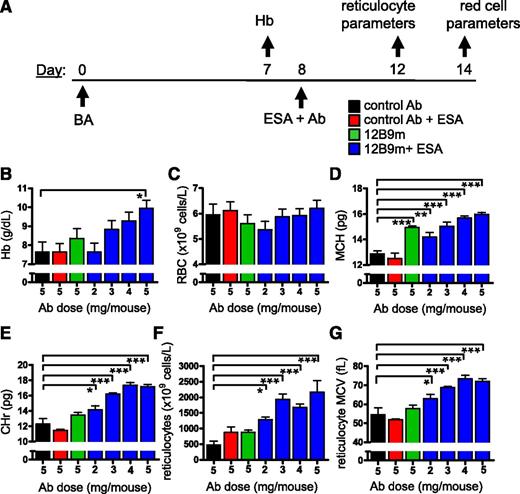
Fully human anti-hepcidin Ab 12B9m effectively treated ESA-refractory anemia in a modified BA model. (A) Experimental scheme detailing administration time of intraperitoneal BA (3 × 108 particles per mouse), intravenous Ab (range of doses), and intravenous ESA (300 µg/kg Epoetin alfa [∼60 000 IU/kg; Amgen]) with the 2 Hb measurement times indicated. (B) BA method modification allowed absolute comparison of Hb values between groups at day 14. Hb values shown at day 0 reflected values of 5 untreated strain-matched mice. Hep2 mice fully backcrossed into a C57BL/6 background (Hep2-C57BL/6) treated with BA had Hb values determined at day 7 and were then distributed between groups such that each treatment group had a similar Hb range resulting in an equivalent mean Hb value for each group. Mice were then exposed to different treatments on day 8, and Hb response to treatment was determined on day 14. Only groups treated with 5 mg of Ab are shown (n = 5 mice per group). (C) Demonstration of dose response to 12B9m treatment. Bar graph detailing the full data set of day 14 Hb values for the study shown in panel B (n = 5 mice per group). Statistical comparisons against control Ab group are shown (1-way analysis of variance [ANOVA] with Dunnett’s post hoc test). **P < .01; ***P < .001. All results are shown as mean ± standard error of the mean (SEM).
Fully human anti-hepcidin Ab 12B9m effectively treated ESA-refractory anemia in a modified BA model. (A) Experimental scheme detailing administration time of intraperitoneal BA (3 × 108 particles per mouse), intravenous Ab (range of doses), and intravenous ESA (300 µg/kg Epoetin alfa [∼60 000 IU/kg; Amgen]) with the 2 Hb measurement times indicated. (B) BA method modification allowed absolute comparison of Hb values between groups at day 14. Hb values shown at day 0 reflected values of 5 untreated strain-matched mice. Hep2 mice fully backcrossed into a C57BL/6 background (Hep2-C57BL/6) treated with BA had Hb values determined at day 7 and were then distributed between groups such that each treatment group had a similar Hb range resulting in an equivalent mean Hb value for each group. Mice were then exposed to different treatments on day 8, and Hb response to treatment was determined on day 14. Only groups treated with 5 mg of Ab are shown (n = 5 mice per group). (C) Demonstration of dose response to 12B9m treatment. Bar graph detailing the full data set of day 14 Hb values for the study shown in panel B (n = 5 mice per group). Statistical comparisons against control Ab group are shown (1-way analysis of variance [ANOVA] with Dunnett’s post hoc test). **P < .01; ***P < .001. All results are shown as mean ± standard error of the mean (SEM).
As previously shown for both Hep1 and Hep2 mice,13 treatment with ESA did not appreciably affect the development of anemia (Figure 2B). Treatment with 5 mg of 12B9m in combination with ESA led to a stable Hb value from day 7 to 14. Doses of 3, 4, or 5 mg 12B9m per mouse in combination with ESA led to an increase in Hb compared with control Ab alone (Figure 2C). The 12B9m monotherapy also significantly increased Hb compared with the control Ab.
Treatment with 12B9m improved iron-dependent red cell characteristics
To explore the mechanism by which 12B9m treatment increased overall Hb, the effect of 12B9m on a range of red cell parameters and early red cell (reticulocyte) characteristics was examined (schema detailed in Figure 3A). Because all mouse strains performed similarly in terms of response to BA-mediated inflammation and Hb response to hepcidin neutralization and ESA treatment, Hep1 mice were used for all subsequent analyses. Two parallel experiments were carried out. One was harvested at day 14 to examine mature red cells (Figure 3B-D), and 1 was harvested at day 12 to examine reticulocyte parameters (Figure 3E-G). This was necessary because phlebotomy at day 12 post-BA treatment to profile reticulocytes at the optimal production point would induce erythropoiesis and influence results observed at day 14.
Treatment with 12B9m led to more effective red cell hemoglobinization and aided in reticulocyte production. (A) Experimental scheme detailing administration time of intraperitoneal BA (3 × 108 particles per mouse), intravenous Ab at doses stated, and intravenous ESA (300 µg/kg Epoetin alfa) in Hep1 mice. Hb was monitored on day 7 and used to assign mice to groups as in Figure 2. Hb (B), RBC number (C), and mean corpuscular Hb (MCH) (D) were measured from 1 study on day 14 (n = 4-5 mice per group). Reticulocyte mean corpuscular Hb (CHr) (E), reticulocyte number (F), and reticulocyte mean corpuscular volume (MCV) (G) were measured from an analogous study on day 12 (n = 4-5 mice per group). Statistical comparisons against control Ab group are shown (1-way ANOVA with Dunnett’s post hoc test). *P < .05; **P < .01; ***P < .001. All results are shown as mean ± SEM.
Treatment with 12B9m led to more effective red cell hemoglobinization and aided in reticulocyte production. (A) Experimental scheme detailing administration time of intraperitoneal BA (3 × 108 particles per mouse), intravenous Ab at doses stated, and intravenous ESA (300 µg/kg Epoetin alfa) in Hep1 mice. Hb was monitored on day 7 and used to assign mice to groups as in Figure 2. Hb (B), RBC number (C), and mean corpuscular Hb (MCH) (D) were measured from 1 study on day 14 (n = 4-5 mice per group). Reticulocyte mean corpuscular Hb (CHr) (E), reticulocyte number (F), and reticulocyte mean corpuscular volume (MCV) (G) were measured from an analogous study on day 12 (n = 4-5 mice per group). Statistical comparisons against control Ab group are shown (1-way ANOVA with Dunnett’s post hoc test). *P < .05; **P < .01; ***P < .001. All results are shown as mean ± SEM.
Hb response of Hep1 mice to 12B9m treatment (Figure 3B) was similar to that shown in Hep2 mice (Figure 2). Treatment-related Hb increase was not driven by an increase in the total number of RBCs (Figure 3C). The MCH was increased in all mice treated with 12B9m, however, indicating more efficient hemoglobinization of red cells produced during the previous 6 days (Figure 3D). A similar increase in MCV was also seen in all groups treated with 12B9m (data not shown). These data are consistent with the effect of short hairpin RNA–mediated hepcidin suppression on MCV and MCH in AI mice.13
Neither ESA treatment nor 12B9m monotherapy led to a significant increase in CHr (Figure 3E). A combination of 12B9m and ESA did increase CHr in a dose-dependent manner. As observed previously,13 ESA treatment did not increase reticulocyte production in BA-treated mice (Figure 3F). Reticulocyte count increased in response to 12B9m and ESA cotreatment, consistent with results from short hairpin RNA–mediated hepcidin suppression combined with ESA treatment.13 Reticulocyte MCV was also increased by combination treatment (Figure 3G). Taken together, these results indicated that treatment with 12B9m and ESA allowed production of effectively hemoglobinized new red cells despite the presence of inflammation.
Treatment with 12B9m did not affect inflammatory cytokine or erythropoietin production in AI mice
To investigate if 12B9m treatment could affect Hb response by modulating inflammatory status, Hep1 mice were treated with BA and 12B9m concurrently, and cytokine concentrations were measured in the serum 6 hours later (peak period of cytokine response to BA treatment13 ). As observed previously, BA treatment led to a significant increase in interleukin (IL) 6 (Figure 4A). No difference in IL-6 concentration was observed between AI mice treated with 12B9m or control Ab, indicating that hepcidin neutralization did not affect IL-6 production. Similar results were observed for IL-1β, tumor necrosis factor α, and granulocyte macrophage–colony-stimulating factor (Figure 4B-D, respectively).
Treatment with 12B9m did not impact inflammatory cytokine or erythropoietin production in mice with AI. Hep1 mice were treated with either intraperitoneal saline or BA (3 × 108 particles per mouse) and intravenous 12B9m or control Ab (5 mg per mouse). Changes in serum concentration of IL-6 (A), IL-1β (B), TNF-α (C), and GM-CSF (D) were measured 6 hours after treatment (n = 3 mice per group). Statistical comparisons against BA + control Ab group are shown (1-way ANOVA with Dunnett’s post hoc test). ***P < .001. (E) In a parallel study, endogenous mouse erythropoietin (mEPO) concentration was measured at days 0, 7, 14, 21, and 28 (n = 4-6 mice per group per time point; 1 value in saline treatment group at day 14 was excluded because the value was >10-fold higher than all other values for saline treatment). Statistical comparisons against BA + control Ab group over time were conducted (2-way ANOVA with Bonferroni post hoc test; no significance). (F) Repeat study examining mEPO at day 14 to confirm results (n = 2-5 mice per group per time point). Statistical comparisons against BA + control Ab group were conducted (1-way ANOVA with Dunnett’s post hoc test; no significance). Results are shown as mean ± SEM. GM-CSF, granulocyte macrophage–colony-stimulating factor; TNF-α, tumor necrosis factor.
Treatment with 12B9m did not impact inflammatory cytokine or erythropoietin production in mice with AI. Hep1 mice were treated with either intraperitoneal saline or BA (3 × 108 particles per mouse) and intravenous 12B9m or control Ab (5 mg per mouse). Changes in serum concentration of IL-6 (A), IL-1β (B), TNF-α (C), and GM-CSF (D) were measured 6 hours after treatment (n = 3 mice per group). Statistical comparisons against BA + control Ab group are shown (1-way ANOVA with Dunnett’s post hoc test). ***P < .001. (E) In a parallel study, endogenous mouse erythropoietin (mEPO) concentration was measured at days 0, 7, 14, 21, and 28 (n = 4-6 mice per group per time point; 1 value in saline treatment group at day 14 was excluded because the value was >10-fold higher than all other values for saline treatment). Statistical comparisons against BA + control Ab group over time were conducted (2-way ANOVA with Bonferroni post hoc test; no significance). (F) Repeat study examining mEPO at day 14 to confirm results (n = 2-5 mice per group per time point). Statistical comparisons against BA + control Ab group were conducted (1-way ANOVA with Dunnett’s post hoc test; no significance). Results are shown as mean ± SEM. GM-CSF, granulocyte macrophage–colony-stimulating factor; TNF-α, tumor necrosis factor.
The effect of hepcidin on endogenous erythropoietin production was also examined in independent cohorts at several time points after Ab treatment (Figure 4E-F). In BA-treated Hep1 mice, endogenous erythropoietin production was not increased by day 7, despite the presence of anemia13 (Figure 4E). No significant difference in erythropoietin concentration was detected with or without Ab treatment in 2 independent experiments (Figure 4E-F).
Treatment with 12B9m was effective when given concurrently with or 2 days after ESA
The impact of changing the timing of 12B9m delivery relative to ESA was explored (schema shown in Figure 5A). Concurrent treatment with 12B9m and ESA led to a significant increase in Hb similar to that seen in previous experiments (Figure 5B). Pretreatment of 12B9m relative to ESA did not lead to increased Hb. Concurrent treatment of 12B9m relative to ESA, or posttreatment, both resulted in a significant increase in Hb (Figure 5C).
Treatment with 12B9m administered concurrently with ESA was more effective than 12B9m administered before or after ESA. (A) Experimental scheme detailing administration time of intraperitoneal BA (3 × 108 particles per mouse), intravenous Ab (5 mg per mouse), and intravenous ESA (300 µg/kg Epoetin alfa) in Hep1 mice. Potential Ab treatment times relative to ESA are indicated. Hb was monitored on either day 6 or day 7 as specified and used to assign mice to groups as in Figure 2. Hb was measured again at day 14 in all experiments (n = 5 mice per group). (B) Comparison of day 14 Hb response to 12B9m administration on day 6 (pretreatment) and day 8 (concurrent treatment) relative to ESA. (C) Comparison of day 14 Hb response to 12B9m administration on day 8 (concurrent treatment) and day 10 (posttreatment). Statistical comparisons against control Ab group are shown (1-way ANOVA with Dunnett’s post hoc test). *P < .05; **P < .01. All results are shown as mean ± SEM.
Treatment with 12B9m administered concurrently with ESA was more effective than 12B9m administered before or after ESA. (A) Experimental scheme detailing administration time of intraperitoneal BA (3 × 108 particles per mouse), intravenous Ab (5 mg per mouse), and intravenous ESA (300 µg/kg Epoetin alfa) in Hep1 mice. Potential Ab treatment times relative to ESA are indicated. Hb was monitored on either day 6 or day 7 as specified and used to assign mice to groups as in Figure 2. Hb was measured again at day 14 in all experiments (n = 5 mice per group). (B) Comparison of day 14 Hb response to 12B9m administration on day 6 (pretreatment) and day 8 (concurrent treatment) relative to ESA. (C) Comparison of day 14 Hb response to 12B9m administration on day 8 (concurrent treatment) and day 10 (posttreatment). Statistical comparisons against control Ab group are shown (1-way ANOVA with Dunnett’s post hoc test). *P < .05; **P < .01. All results are shown as mean ± SEM.
Serum iron response to anti-hepcidin Ab treatment was more pronounced in mice with inflammation
To explore the kinetics and duration of response to anti-hepcidin Ab treatment, Hep1 mice with and without inflammation were treated with 12B9m or a control Ab. Mice were administered BA or saline control and then 5 mg of 12B9m or control Ab 7 days later. Serum iron was measured in independent cohorts at 4 hours or 1, 3, or 7 days after Ab administration. In Hep1 mice without inflammation, baseline serum iron concentration was ∼400 µg/dL and was not appreciably affected by 12B9m treatment at any time point sampled (Figure 6A). In Hep1 mice with inflammation, serum iron 7 days after BA treatment was ∼200 µg/dL, and a noticeable increase was seen as soon as 4 hours after 12B9m treatment, reaching significance at 1 day and approaching a return to baseline by 3 days (Figure 6B).
Ab-mediated neutralization of hepcidin led to increased serum iron in mice and cynomolgus monkeys. (A) The 12B9m treatment did not increase serum iron in Hep1 mice without inflammation. Intravenous 12B9m was administered (5 mg per mouse) to Hep1 mice, and serum iron was measured in different cohorts of mice at baseline, 4 hours, 1 day, 3 days, and 7 days (n = 3 mice per group per time point). (B) The 12B9m treatment increased serum iron in Hep1 AI mice. Hep1 mice were treated with intraperitoneal BA (2 × 108 particles per mouse) and then 1 week later with intravenous 12B9m. Serum iron was sampled as specified in panel A. (C) Mouse monoclonal anti-mouse hepcidin Ab 2C10 treatment increased serum iron in normal C57BL/6 mice. Intravenous 2C10 was administered (5 mg per mouse) to C57BL/6 mice, and serum iron was measured in different cohorts at baseline and 1, 2, 3, and 4 days after treatment (n = 4 mice per group per time point). (D) The 2C10 treatment increased serum iron in C57BL/6 AI mice. Mice were treated with intraperitoneal BA (5 × 108 particles per mouse) and then 1 week later with 2C10, and serum iron was sampled as specified in panel C (n = 5 mice per group per time point). (E) A single dose of 12B9m elevated serum iron in cynomolgus monkeys prior to saturation of Ab with hepcidin. Male cynomolgus monkeys were treated with intravenous 12B9m (50 mg/kg), and blood was collected at baseline, 0.5 hours, 4 hours, 1 day, 2 days, 4 days, and 7 days and assayed for 12B9m concentration, total hepcidin concentration (dissociated from Ab prior to measurement), and serum iron (n = 3 monkeys per group). Statistical comparisons against baseline serum iron values are shown (1-way ANOVA with Dunnett’s post hoc test). *P < .05; **P < .01. All results are shown as mean ± SEM. (F) Cynomolgus monkeys treated repeatedly with 12B9m showed a similar serum iron response in the fourth cycle of treatment as in the first. Cynomolgus monkeys (n = 10) were administered intravenous 12B9m (5, 40, or 300 mg/kg) once weekly. Blood was collected at 4 hours, 1 day, 2 days, 4 days ,and 7 days after the first injection (cycle 1) and fourth injection (cycle 4) and assayed for serum iron. Statistical comparisons against control-treated animals are shown (repeated measures ANOVA [RMANOVA] with Bonferroni post hoc test; colored dots represent time points). P < .05. All results are shown as mean ± SEM.
Ab-mediated neutralization of hepcidin led to increased serum iron in mice and cynomolgus monkeys. (A) The 12B9m treatment did not increase serum iron in Hep1 mice without inflammation. Intravenous 12B9m was administered (5 mg per mouse) to Hep1 mice, and serum iron was measured in different cohorts of mice at baseline, 4 hours, 1 day, 3 days, and 7 days (n = 3 mice per group per time point). (B) The 12B9m treatment increased serum iron in Hep1 AI mice. Hep1 mice were treated with intraperitoneal BA (2 × 108 particles per mouse) and then 1 week later with intravenous 12B9m. Serum iron was sampled as specified in panel A. (C) Mouse monoclonal anti-mouse hepcidin Ab 2C10 treatment increased serum iron in normal C57BL/6 mice. Intravenous 2C10 was administered (5 mg per mouse) to C57BL/6 mice, and serum iron was measured in different cohorts at baseline and 1, 2, 3, and 4 days after treatment (n = 4 mice per group per time point). (D) The 2C10 treatment increased serum iron in C57BL/6 AI mice. Mice were treated with intraperitoneal BA (5 × 108 particles per mouse) and then 1 week later with 2C10, and serum iron was sampled as specified in panel C (n = 5 mice per group per time point). (E) A single dose of 12B9m elevated serum iron in cynomolgus monkeys prior to saturation of Ab with hepcidin. Male cynomolgus monkeys were treated with intravenous 12B9m (50 mg/kg), and blood was collected at baseline, 0.5 hours, 4 hours, 1 day, 2 days, 4 days, and 7 days and assayed for 12B9m concentration, total hepcidin concentration (dissociated from Ab prior to measurement), and serum iron (n = 3 monkeys per group). Statistical comparisons against baseline serum iron values are shown (1-way ANOVA with Dunnett’s post hoc test). *P < .05; **P < .01. All results are shown as mean ± SEM. (F) Cynomolgus monkeys treated repeatedly with 12B9m showed a similar serum iron response in the fourth cycle of treatment as in the first. Cynomolgus monkeys (n = 10) were administered intravenous 12B9m (5, 40, or 300 mg/kg) once weekly. Blood was collected at 4 hours, 1 day, 2 days, 4 days ,and 7 days after the first injection (cycle 1) and fourth injection (cycle 4) and assayed for serum iron. Statistical comparisons against control-treated animals are shown (repeated measures ANOVA [RMANOVA] with Bonferroni post hoc test; colored dots represent time points). P < .05. All results are shown as mean ± SEM.
Baseline serum iron concentration in Hep1 mice was appreciably increased compared with both littermate controls and wild-type C57BL/6.13 This raised the possibility that the difference in response to 12B9m treatment seen between inflamed and noninflamed mice was due to the high initial serum iron in Hep1 mice. For this reason, a similar experiment was conducted in C57BL/6 mice using a mouse anti-mouse hepcidin neutralizing Ab (2C10). Treatment of noninflamed mice with 2C10 increased serum iron for 3 days, reaching a maximal concentration of ∼400 µg/dL from a baseline of 200 µg/dL (Figure 6C). When C57BL/6 mice with inflammation were treated with 2C10, an increase of greater magnitude was seen than was observed in mice with no inflammation (maximal concentration of ∼600 µg/dL; Figure 6D). The duration of effect was shorter, however, presumably due to saturation of the Ab.
A relationship was observed between duration of 12B9m response in cynomolgus monkeys and kinetics of Ab saturation
To explore the relationship between 12B9m PK and PD, cynomolgus monkeys were administered 50 mg/kg of intravenous 12B9m. Molar Ab concentration was calculated using a molecular mass of 75 kDa (half total Ab mass) to allow direct comparison of moles of hepcidin-binding sites on the Ab with moles of hepcidin. Total hepcidin concentration included both free hepcidin and hepcidin dissociated from Ab by an acid incubation step.
At the earliest time point sampled after intravenous injection, the 12B9m concentration was already maximal and decreased slowly over time consistent with normal human IgG2 PK14 (Figure 6E). Serum iron concentration increased gradually over time with maximal concentration observed between 1 and 2 days and approached baseline concentration at day 4. During the same time period when serum iron concentration was declining (days 2-4), total hepcidin concentration increased to reach approximately an equimolar ratio with Ab and then exhibited the same kinetics as total Ab concentration,14 suggesting that Ab saturation with hepcidin occurred between days 2 and 4. By day 7, total hepcidin concentration was still extremely high (>5 µM = ∼15 µg/mL). This value was several hundred-fold higher than baseline cynomolgus hepcidin concentration (average of 44 ng/mL in the study shown). Despite the high concentration of hepcidin present, normal serum iron concentration was restored at day 7, suggesting that the hepcidin present was stably complexed with Ab and unable to modulate iron metabolism.
Repeated dosing of 12B9m was effective at modulating cynomolgus serum iron concentration
Effects of repeated 12B9m administration in cynomolgus monkeys were examined. Monkeys were treated with 5, 40, or 300 mg/kg intravenous 12B9m once a week for 4 weeks and compared with untreated controls. Response to the first and fourth cycles was monitored by analysis at several time points postinjection.
A dose response to 12B9m therapy was observed in both the first and fourth cycles, which were similar in intensity and duration (Figure 6F). For monkeys treated with 5 mg/kg 12B9m, a transient increase in serum iron was observed, which was significant at 4 hours and 1 day postdosing in the fourth cycle. For the 40 mg/kg dose group, a difference from baseline was observed in the first 2 days in each cycle. For the 300 mg/kg dose group, all time points were increased over baseline, including all predose points in cycles 2 to 4 (data not shown). This indicated that 300 mg/kg achieved complete target coverage. A subcutaneous group dosed with 300 mg/kg 12B9m exhibited a similar serum iron response to the 300 mg/kg intravenous group.14
Discussion
In the process of assessing fully human anti-hepcidin Abs as potential human therapeutics, we performed studies demonstrating that a rare class of Abs was capable of binding to a second epitope on hepcidin, leading to the establishment of an anti-hepcidin sandwich ELISA. This assay had a similar performance and sensitivity range to the sandwich ELISA previously published.23 Hepcidin has been shown to be a biomarker of potential response to ESA therapy,25 and high hepcidin also appears to limit response to intravenous iron.26,27 For this reason, a hepcidin detection assay could be useful in predicting potential responses to ESA and intravenous iron treatment, in addition to identifying patients who may benefit from an anti-hepcidin Ab treatment.
Activity of fully human anti-hepcidin Ab 12B9m was examined in a mouse model of AI. Using ESA cotreatment, it was possible to increase Hb by as much as 3 to 4 g/dL over a 1 week time span. This represented an increase that was at the limits of possible red cell response in this time frame.28 Ab 12B9m treatment alone was able to increase Hb by 1 to 2 g/dL (statistically significant in some but not all experiments, with a larger response overall generally seen in Hep2 mice than in Hep1 mice). Strain differences may potentially be driven by residual expression of mouse hepcidin 2 in Hep1 mice leading to the presence of an Ab sink. The sum total of experiments with both strains suggested, however, that single-agent treatment with an anti-hepcidin therapy may constitute an appropriate therapy for AI in patients not able to receive ESA, such as patients with the anemia of cancer. Indeed, because in a clinical situation a controlled rate of Hb increase has been recommended (1 g/dL per 2 weeks), single-agent activity of an anti-hepcidin therapy or anti-hepcidin therapy combined with low-dose ESA may be ideal.
The hypothesis for the mechanism of action of anti-hepcidin therapy was that iron mobilization increased the mean cell volume and Hb content in the fraction of red cells produced as a consequence of treatment and therefore increased overall Hb. Analysis of reticulocytes at the peak production time after treatment (day 4) confirmed this hypothesis. Reticulocyte count was not increased by either ESA or anti-hepcidin therapy alone but required both. Values for reticulocyte Hb and MCV were actually somewhat higher than normal as a result of therapy (data not shown). These effects were maximal when ESA and anti-hepcidin therapy were combined. Although these effects did not translate to an increase in overall RBC numbers, they did lead to increases in MCH and MCV. Hepcidin neutralization did not appear to affect red cell production by other mechanisms such as downmodulation of inflammation or affecting endogenous erythropoietin production, suggesting that all effects observed in the inflammatory model were due to enhanced iron incorporation and also potentially to increased red cell precursor survival (inferred by greater reticulocyte response seen).
Several dosing scenarios for the combination of ESA and anti-hepcidin therapy were examined. Dosing anti-hepcidin therapy concomitantly with ESA or after ESA treatment was more effective than dosing 2 days prior to treatment. This suggests that the Ab-mediated serum iron peak from pretreatment did not coincide with maximal iron utilization by new red cells but that it did for concurrent or posttreatment. This is consistent with the expression of transferrin receptor, which is present at a high concentration at later stages of erythroid differentiation, facilitating a “later” benefit of iron loading.29
One of the potential drawbacks to an anti-hepcidin therapy is that it involves targeting a ligand that is under homeostatic control. Decrease in circulating hepcidin would increase circulating iron, leading to more hepcidin production. This rebound hepcidin production could conceivably saturate the Ab and negate its effect. In line with this theory, hepcidin knock-in mice treated with an anti-hepcidin Ab did show little or no benefit in terms of serum iron response, and wild-type mice treated with a mouse anti-hepcidin Ab only showed a modest increase in serum iron. However, in both strains of mice with inflammation, a dramatic serum iron response to treatment was seen. It could be speculated that this was due to the decreased serum iron at the time of treatment, and hence hepcidin homeostatic regulation was not triggered until “normal” serum iron is reached. Another possible explanation is that homeostatic control is absent in mice with inflammation. Measurement of free hepcidin was attempted to clarify this question but could not be detected in the presence of Ab treatment. In the process of analysis, the huge molar excess of Ab-complexed hepcidin led to dissociation and hence inaccurate “free” hepcidin measurement.
A high-affinity anti-hepcidin Ab was chosen partly to minimize the potential liability of Ab-ligand dissociation in vivo. In vivo dissociation was subsequently tested for in a PK/PD study. When the total hepcidin concentration (free and bound hepcidin) reached the same approximate molarity as the hepcidin-binding sites on the Ab, hepcidin assumed Ab-like PK, indicating that the Ab was fully complexed. The therapeutic effect of the Ab on serum iron was then lost. Similar results were also observed with an anti-hepcidin spiegelmer.30 Although “free” hepcidin could not be accurately measured, the fact that serum iron returned to normal despite the presence of an ∼400-fold increased hepcidin concentration strongly suggests that Ab-ligand dissociation did not limit activity of 12B9m. Repeated exposures of cynomolgus monkeys to 12B9m treatment did not markedly change this result, with the fourth cycle of weekly dosing resembling the first cycle. Consistent with the lower circulating concentration of hepcidin in cynomolgus monkeys (20-50 ng/mL) compared with mice (100-200 ng/mL) and the slower glomerular filtration rate, the production rate of hepcidin in cynomolgus monkey is estimated to be ∼12-fold lower than that in mice. Hence, a dose of 5 mg/kg in normal cynomolgus monkeys appeared to give comparable results with 200 mg/kg in normal mice. Because the calculated production rate of hepcidin in noninflamed patients is ∼100-fold less than in mice,13 it is presumed that activity of the Ab would be seen at a lower dose in humans than in cynomolgus monkeys. Given the increase in activity in mice with inflammation compared with normal mice, it could be contemplated that even greater serum iron changes would be seen in patients with inflammation or impaired hepcidin clearance than in normal human volunteers.
In summary, dosing of 12B9m sufficient to elevate serum iron for a few days either alone or in combination with ESA treatment produced a meaningful elevation in Hb in a mouse model of AI. In addition, cynomolgus monkey data suggested that repeated treatments with an anti-hepcidin was effective and required lower doses than in mice. Based on this data, treatment of anemia patients with fully human anti-hHepc Ab 12B9m may be practicable and represent an appropriate treatment of AI.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jeanne Sloan, Adrienne Augustic, Marcus Soto, and Manuel Ponce for in vivo assistance; Vimal Patel and Linh Tran for aid in hepcidin measurement; and Raheem Khaja for help with endogenous erythropoietin determination.
This work was supported by by Amgen Inc.
Authorship
Contribution: K.S.C., B.H., H.S.-M., I.F., C.K., P.R., A.W., and S.S. performed experiments and interpreted data; C.G.B. and G.M. aided in study interpretation and context; and K.S.C. and B.J.S. designed and interpreted the work, wrote the manuscript, and are responsible for the integrity of the work as a whole.
Conflict-of-interest disclosure: All authors are current or former employees of Amgen Inc.
Correspondence: Keegan Cooke, Mailstop 15-2-A, 1 Amgen Center Drive, Thousand Oaks, CA 91320; e-mail: kcooke@amgen.com.

![Figure 1. Characterization of hepcidin-binding Abs and development of a sandwich ELISA. (A) Polyclonal Abs against human hepcidin demonstrated that construction of a sandwich ELISA was possible. Each polyclonal Ab (4364 and 4366) was tested as both the capture Ab (eg, 4364 bound to ELISA plate) and detection Ab (4364 conjugated to horseradish peroxidase for detection). (B) The ability of 2 Abs to bind to hepcidin simultaneously was profiled by Biacore. Representative plot showing a panel of Abs, some of which bound to Ab 19D12 coated on a chip and treated with hepcidin. Binding of the second Ab was demonstrated by an increase in detected mass (increase in relative units [RU] of binding). (C) Diagram categorizing hepcidin-binding Abs into classes based on ability to recognize overlapping or distinct epitopes on hepcidin. Solid line indicates classes that could bind hepcidin simultaneously. (D) Comparison of performance of anti-human hepcidin sandwich ELISA with hepcidin detection by LC-MS/MS. Serum hepcidin concentrations from CKD patient samples were measured in both assays and compared (n = 46). Hepcidin ELISA (x-axis) and LC-MS/MS (y-axis).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/17/10.1182_blood-2013-06-505792/4/m_3054f1.jpeg?Expires=1763812669&Signature=hPbeV5zVzSM0j66QmS9MASxKZPtTthruKVgIz344HZxaabJMu76j~195nSfBrT~1FSoNqKNsHLA-Se7Ym~FAZNW8lfK2COq~RGhrXnEwniwy54DTewIh9t7-ZCJ6ldIMZj6msYs9DioS171LFcdbLgzNpZ0tMX-2JVoxpaq0R4FRe14OUVQdEjoTc3QrDd3ENJFxESG89KnOeToLOZLnMQraR7Y3TQXXxU-2qL5hndDPzypvfXjs9nG~2Hm2eNH8LPNgyJep1R1Z4vkDiD6czu1bJgvK4R3K4JlzyBOg6wwA8Y64hCpyv1qNA3HLuiQxjidTRGrTmHBhclTnwGkaqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Fully human anti-hepcidin Ab 12B9m effectively treated ESA-refractory anemia in a modified BA model. (A) Experimental scheme detailing administration time of intraperitoneal BA (3 × 108 particles per mouse), intravenous Ab (range of doses), and intravenous ESA (300 µg/kg Epoetin alfa [∼60 000 IU/kg; Amgen]) with the 2 Hb measurement times indicated. (B) BA method modification allowed absolute comparison of Hb values between groups at day 14. Hb values shown at day 0 reflected values of 5 untreated strain-matched mice. Hep2 mice fully backcrossed into a C57BL/6 background (Hep2-C57BL/6) treated with BA had Hb values determined at day 7 and were then distributed between groups such that each treatment group had a similar Hb range resulting in an equivalent mean Hb value for each group. Mice were then exposed to different treatments on day 8, and Hb response to treatment was determined on day 14. Only groups treated with 5 mg of Ab are shown (n = 5 mice per group). (C) Demonstration of dose response to 12B9m treatment. Bar graph detailing the full data set of day 14 Hb values for the study shown in panel B (n = 5 mice per group). Statistical comparisons against control Ab group are shown (1-way analysis of variance [ANOVA] with Dunnett’s post hoc test). **P < .01; ***P < .001. All results are shown as mean ± standard error of the mean (SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/17/10.1182_blood-2013-06-505792/4/m_3054f2.jpeg?Expires=1763812669&Signature=zCwWO3dyxJYDw3EIHTML1Lyslv481P2NPa3iBnWyU45p4xH4qV7j8-gN2rboMa40Lc9rbzwlt57pU2c1XeD-ZBuaJBjKD7pwBBt4-xTGGcJBTNSIz5WyBU4kCRMVFDbfFIeanZQ1uEh2WcuZzup2vtaGRex1HMm8yWG-4rMjlZ4IoD0AYxW6AL6fvMTVZ1ROFk-PB~dlNH12q3UandKC9rCoPX6owUPdi-pU0XX448UkqwjB~3BaGphmzcjflpUcRd~lNw5xGxDiEYx58b1p-qnm-A3fY8fLH-6Eg5CMDe8qgaNn5h~E7i~4fvhJ23Le2WF9ddJqcKOoXnha-ihFqA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 6. Ab-mediated neutralization of hepcidin led to increased serum iron in mice and cynomolgus monkeys. (A) The 12B9m treatment did not increase serum iron in Hep1 mice without inflammation. Intravenous 12B9m was administered (5 mg per mouse) to Hep1 mice, and serum iron was measured in different cohorts of mice at baseline, 4 hours, 1 day, 3 days, and 7 days (n = 3 mice per group per time point). (B) The 12B9m treatment increased serum iron in Hep1 AI mice. Hep1 mice were treated with intraperitoneal BA (2 × 108 particles per mouse) and then 1 week later with intravenous 12B9m. Serum iron was sampled as specified in panel A. (C) Mouse monoclonal anti-mouse hepcidin Ab 2C10 treatment increased serum iron in normal C57BL/6 mice. Intravenous 2C10 was administered (5 mg per mouse) to C57BL/6 mice, and serum iron was measured in different cohorts at baseline and 1, 2, 3, and 4 days after treatment (n = 4 mice per group per time point). (D) The 2C10 treatment increased serum iron in C57BL/6 AI mice. Mice were treated with intraperitoneal BA (5 × 108 particles per mouse) and then 1 week later with 2C10, and serum iron was sampled as specified in panel C (n = 5 mice per group per time point). (E) A single dose of 12B9m elevated serum iron in cynomolgus monkeys prior to saturation of Ab with hepcidin. Male cynomolgus monkeys were treated with intravenous 12B9m (50 mg/kg), and blood was collected at baseline, 0.5 hours, 4 hours, 1 day, 2 days, 4 days, and 7 days and assayed for 12B9m concentration, total hepcidin concentration (dissociated from Ab prior to measurement), and serum iron (n = 3 monkeys per group). Statistical comparisons against baseline serum iron values are shown (1-way ANOVA with Dunnett’s post hoc test). *P < .05; **P < .01. All results are shown as mean ± SEM. (F) Cynomolgus monkeys treated repeatedly with 12B9m showed a similar serum iron response in the fourth cycle of treatment as in the first. Cynomolgus monkeys (n = 10) were administered intravenous 12B9m (5, 40, or 300 mg/kg) once weekly. Blood was collected at 4 hours, 1 day, 2 days, 4 days ,and 7 days after the first injection (cycle 1) and fourth injection (cycle 4) and assayed for serum iron. Statistical comparisons against control-treated animals are shown (repeated measures ANOVA [RMANOVA] with Bonferroni post hoc test; colored dots represent time points). P < .05. All results are shown as mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/17/10.1182_blood-2013-06-505792/4/m_3054f6.jpeg?Expires=1763812669&Signature=io-5RnjY~I-FxiyaoG8PB6cvHp6uBGUFuoEQzfYSfsakbbFG2qo~-6tJBDwHpWtcc~R5q6ImBMor3com1XfBevUkOVOR7n-RfhBj6mRQmcbi3Hg8PUBR-MD3OMNbQLSPeSr6M58Btdgbr4Vfa4Yema9CeLyGrQTjdjwn8pSveh0WK4XtDXG8GVgyrotCUbbVvIwxCziu1zPiyR8ioXjkxDc5b8o2zYj03EHSTzQ3-BBuVCWSRKAKfzaDCCt-Ko9ZMOC~MoL9kOJTjVBr7M1oP4347QeoHklyI0iKmGHpt~T6-78-ud5KI0QPa6yBz4lW8atc1FXmQ0Pd7JvZ~KIVbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)