Key Points
Immunologic and virologic factors are associated with monoclonal gammopathy persistence in HIV-infected patients.
B lymphocytes activation and EBV replication are key features of monoclonal gammopathy.
Abstract
A high prevalence of monoclonal gammopathy (MG) has been observed in HIV-infected patients. We explored the conditions associated with long-term persistence of serum monoclonal protein (M protein) in HIV-infected patients on antiretroviral therapy (ART). Of 21 patients with MG, M protein disappeared in 12 patients (58%) over 5 years of ART. Higher level of serum γ-globulin and higher percentages of circulating plasmablasts and plasma cells were observed in patients with persistent MG compared with patients with transient MG. MG persistence was associated with the cumulative time of detectable plasma HIV RNA after ART initiation, detection of Epstein-Barr virus (EBV) DNA in plasma, and a high level of EBV DNA in B cells. Poor control of HIV replication and detectable EBV replication in plasma were both associated with long-term MG persistence in patients on ART. In the case of viral control, MG associated with HIV infection is usually transient.
Introduction
HIV induces numerous impairments in B-cell function.1 Polyclonal hypergammaglobulinemia is one of the major manifestations of B-cell deficiencies associated with HIV replication.2 Monoclonal and oligoclonal proteins detected in serum are also frequently observed in HIV-infected patients.3 With the advent of the widespread use of combination antiretroviral treatment (ART), it has become clear that long-term effective ART dramatically reverses many B-cell abnormalities, including polyclonal activation, autoimmune response, plasmacytosis, and hypergammaglobulinemia.4-6
In HIV-infected persons, the prevalence of monoclonal gammopathy (MG) is estimated to be between 3% and 26%3 and occurs at a younger age compared with the general population, in which the prevalence is estimated to be 3.2% in persons over 50 years old and 5.3% in those over 70 years old.7 The significance and evolution of MG after ART initiation remains unclear.
Previous studies reported that the impairment of specific T-cell function in HIV-infected individuals is associated with an increase in Epstein-Barr virus (EBV) DNA load in peripheral blood mononuclear cells.8 B-cell hyperactivation and terminal differentiation into plasmablasts and plasma cells induce EBV reactivation and contribute to the increase of its reservoir.9,10 The close relationship between EBV and lymphoproliferative disorders may include MG in HIV-infected patients. We hypothesized that the persistence of MG in HIV-infected individuals requires continuous B-cell stimulation fueled by both HIV and EBV replications.
In the present study, we investigated whether immune activation and HIV and EBV replication were associated with the long-term persistence of MG in HIV-infected patients on ART exhibiting a monoclonal band at the time of HIV diagnosis.
Methods
Patients and study design
Twenty-one patients in whom monoclonal protein (M protein) was identified by combined protein electrophoresis (PEP) and immunofixation were included between 1998 and 2005. All patients were treated and regularly followed up for HIV therapeutic monitoring at the Infectious Disease Department of the Montpellier University Teaching Hospital. MG was detected before treatment initiation. Concentrations of the M protein ranged from unquantifiable (15/21 cases) to 19.3 g/L, with a median of 3.1 g/L when quantifiable. Fifteen HIV-infected subjects without monoclonal band were included as controls. The long-term persistence of MG was controlled in 2011 after at least 5 years of ART by PEP (Capillarys 2; SEBIA, Evry, France) and immunofixation (Hydrasys 2 [SEBIA]). We defined MG as “persistent” when the monoclonal peak remained detectable and confirmed, and as “transient” when the monoclonal peak disappeared. Patients’ characteristics and treatments are presented in Table 1. EBV DNA in B cell, plasma EBV DNA, and ex vivo EBV-associated B cells were analyzed as previously described10,11 and are detailed in the supplemental Data (available on the Blood website). This study was conducted in accordance with the Declaration of Helsinki. The study protocol and sample collection were registered at the French Health Ministry (number DC-2008-417).
Statistical analysis
The Wilcoxon rank-sum test was performed using the R software to compare median values of nonparametric variables. Fisher’s exact test was used in the case of proportion comparisons. The exact logistic regression and multivariate analysis were performed to search independent associations between the persistence of the monoclonal peak and HIV RNA viremia, EBV DNA load in plasma, gain of CD4+ T cells, hepatitis B and C coinfection, and cytomegalovirus (CMV) reactivation.
Results and discussion
Of the 21 patients with detectable MG by PEP, which was confirmed by immunofixation before ART initiation, 9 (42%) patients still harbored MG after a prolonged period on ART (≥5 years), whereas the monoclonal peak disappeared for the other 12 (58%) patients. This observation reveals a labile characteristic of MG associated with HIV infection and ART in contrast to the MG detected in monoclonal gammopathy of undetermined significance (MGUS), myeloma, or lymphoma, in which MG is maintained lifelong.12
In line with previous reports,7 we observed that MG occurs at a younger age among HIV-infected individuals when compared with the general population (Table 1). No differences were found at baseline between the transient and persistent MG groups for plasma HIV RNA load and high expression of CD38 on CD8+ T lymphocytes. Most of the patients studied had low CD4+ T-cell nadir, but there was no significant difference in the nadir of CD4+ T-cell count or in CD4+ T-cell replenishment over the ART period between patients with persistent MG vs transient MG. The exact logistic regression showed that the persistence of MG was associated with the number of years of detectable HIV RNA load during therapy (odds ratio [OR] = 2.2, 95% confidence interval [CI], 1.1-5.9; P = .01; supplemental Table 1). After a prolonged period on ART, a twofold higher percentage of CD8+ T-cell activation (% of CD38bright CD8+ T cells) was observed in the persistent MG group compared with the transient MG group, although the difference was not significant (P = .06). Polyclonal CD8+ T-cell hyperactivation is known to be a robust marker of the immune activation driven by HIV and an independent predictive marker of HIV disease progression.13,14
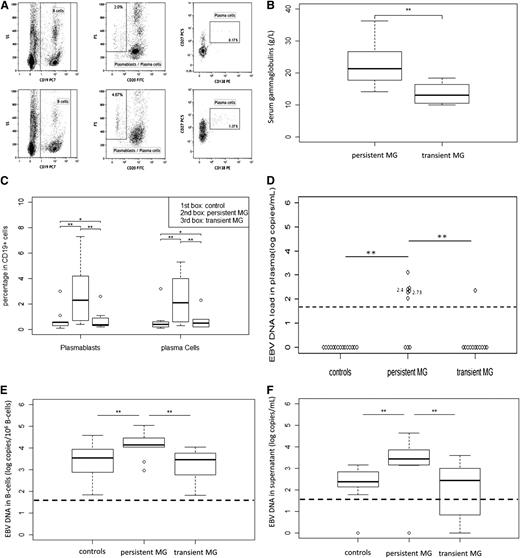
The persistence of the MG was accompanied by a higher serum immunoglobulin level (P = .003) (Figure 1B), mainly restricted to the immunoglobulin G (IgG) isotype. Hypergammaglobulinemia was observed in 11 of 21 patients (52%), namely 8 of 9 (89%) in the persistent MG group and 3 of 12 (25%) in the transient MG group (P < .001). High frequencies of circulating plasmablasts and plasma cells were observed in the persistent MG group (P = .01 and .02, respectively), (Figure 1A,C). These results provide evidence that mature B cells are prompted to undergo terminal differentiation into antibody-producing cells and may be indicative of persistent B-cell activation through systemic antigenic stimulation.15
Analysis of circulating plasmablast and plasma cells, polyclonal immunoglobulin levels, and EBV DNA levels in HIV-infected patients with transient MG or persistent MG. (A) Gating strategy and phenotypes of circulating plasmablast and plasma cells. Circulating cells were stained with CD19, CD20, CD27, and CD138 monoclonal antibodies. Plasmablasts were identified as CD19+, CD20–, CD27+, and CD138– cells. Plasma cells were identified as CD19+, CD20–, CD27+, and CD138+ cells. Plasma cells are large cells expressing higher levels of CD27 than memory B cells. Circulating plasma cells represented 0.1% to 5% of B cells in HIV-infected patients. (B) Plasmablasts and plasma cells in the blood of transient MG, persistent MG, and control patients. Percentage of plasmablasts and plasma cells among CD19+ B cells were determined. (C) Polyclonal immunoglobulin levels in patients with persistent and transient MG. Serum levels of gammaglobulins were determined by protein electrophoresis at least after 5 years of follow-up under ART. (D) EBV DNA load in plasma (Log copies/mL). (E) EBV DNA load in B cells (Log copies/106 cells), numbers near plots indicate corresponded HIV RNA load. (F) EBV DNA in B-cell culture supernatant after 48 hours of culture. The ends of the boxes indicate the 25th and 75th percentiles, with a line at the median; error bars represent the 10th and 90th percentiles. **P < .05; *P > .05.
Analysis of circulating plasmablast and plasma cells, polyclonal immunoglobulin levels, and EBV DNA levels in HIV-infected patients with transient MG or persistent MG. (A) Gating strategy and phenotypes of circulating plasmablast and plasma cells. Circulating cells were stained with CD19, CD20, CD27, and CD138 monoclonal antibodies. Plasmablasts were identified as CD19+, CD20–, CD27+, and CD138– cells. Plasma cells were identified as CD19+, CD20–, CD27+, and CD138+ cells. Plasma cells are large cells expressing higher levels of CD27 than memory B cells. Circulating plasma cells represented 0.1% to 5% of B cells in HIV-infected patients. (B) Plasmablasts and plasma cells in the blood of transient MG, persistent MG, and control patients. Percentage of plasmablasts and plasma cells among CD19+ B cells were determined. (C) Polyclonal immunoglobulin levels in patients with persistent and transient MG. Serum levels of gammaglobulins were determined by protein electrophoresis at least after 5 years of follow-up under ART. (D) EBV DNA load in plasma (Log copies/mL). (E) EBV DNA load in B cells (Log copies/106 cells), numbers near plots indicate corresponded HIV RNA load. (F) EBV DNA in B-cell culture supernatant after 48 hours of culture. The ends of the boxes indicate the 25th and 75th percentiles, with a line at the median; error bars represent the 10th and 90th percentiles. **P < .05; *P > .05.
Memory B lymphocytes are the main reservoir of EBV, and their differentiation into plasma cells can reactivate this reservoir. As is shown in Figure 1D, EBV DNA was detectable in the plasma of 1 of 12 patients in transient MG and in 0 of 15 of the HIV-infected controls, whereas 6 of 9 patients with persistent MG on ART (66%) exhibited quantifiable EBV DNA in plasma (P < .001). Multivariate analysis showed that the EBV DNA level in plasma was associated with persistence of MG, independent of detectable HIV RNA load and CD4+ T-cell replenishment (OR = 18.6; 95% CI, 2.02-∞, P = .007, supplemental Table 1). The EBV DNA B-cell reservoir was fivefold higher in persistent MG (P = .006) and fourfold higher than controls (P = .007) (Figure 1E). EBV DNA spontaneously produced by infected B cells maintained in culture for 48 hours was more than tenfold higher in persistent MG compared with transient MG (P = .002) and compared with controls (P = .001) (Figure 1F). Altogether, these observations indicate that: (1) markers of EBV replication; (2) number of EBV latently infected B cells; and (3) EBV-infected cells primed to enter and complete the EBV cycle were associated with the persistence of MG.
In addition to the young age at the time of MG diagnosis, virus stimulation of B cells and reversion to a transient state are 2 important characteristics of HIV-associated MG. In the general population, MG occurrence is related to molecular pathogenic events such as IgH translocations, aneuploidy, chromosome 13 deletion, and dysregulation of a CYCLIN D gene.16,17 Chronic bacterial antigen stimulation has also been associated with an increased risk of MGUS and multiple myeloma.18,19 Reduction in the level of M protein after initiation of combined ART has been reported previously.20 In HIV-infected individuals, ongoing EBV replication may fuel persistent lymphocyte activation and consequently be part of an amplification loop that adversely affects the host immune response21,22 and favors MG persistence. MG associated with HIV infection may share common features with posttransplant MG because EBV reactivation and increased frequency of EBV latently infected B cells are observed in these cases as well.23,24 These features make the physiopathologic mechanisms behind HIV-associated MG different from the MGUS observed in the general population and closer to the MG observed in posttransplant patients.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sophie Bendriss for the technical assistance and Audrey De Jong for statistical analysis.
This study was supported by student grant from Infectiopole Sud.
Authorship
Contribution: D.E.O. designed the study, performed research, analyzed data, and wrote the paper; A.M., J.-P.V., P.V.d.P., and E.T. designed the study and wrote the paper; and J.V., M.-L.C., N.N., R.C., Y.A.T., S.B., K.B., V.F., and J.R. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Eric Ouedraogo, Institute of Research in Biotherapy, CHU Montpellier Hôpital Saint-Eloi, 80, av. Augustin Fliche, 34295 Montpellier Cedex 5, France; e-mail: daverid32@yahoo.fr.