Key Points
Epratuzumab induces the reduction of multiple B-cell antigen receptor–modulating proteins on the surface of B cells via their trogocytosis to effector cells.
Modulation of B cells by trogocytosis of key regulatory proteins may be an important mechanism of immunotherapy of autoimmune disease.
Abstract
Epratuzumab, a humanized anti-CD22 antibody, is currently in clinical trials of B-cell lymphomas and autoimmune diseases, demonstrating therapeutic activity in non-Hodgkin lymphoma (NHL) and systemic lupus erythematosus (SLE). Thus, epratuzumab offers a promising option for CD22-targeted immunotherapy, yet its mechanism of action remains poorly understood. Here we report for the first time that epratuzumab promptly induces a marked decrease of CD22 (>80%), CD19 (>50%), CD21 (>50%), and CD79b (>30%) on the surface of B cells in peripheral blood mononuclear cells (PBMCs) obtained from normal donors or SLE patients, and of NHL cells (Daudi and Raji) spiked into normal PBMCs. Although some Fc-independent loss of CD22 is expected from internalization by epratuzumab, the concurrent and prominent reduction of CD19, CD21, and CD79b is Fc dependent and results from their transfer from epratuzumab-opsonized B cells to FcγR-expressing monocytes, natural killer cells, and granulocytes via trogocytosis. The findings of reduced levels of CD19 are implicative for the efficacy of epratuzumab in autoimmune diseases because elevated CD19 has been correlated with susceptibility to SLE in animal models as well as in patients. This was confirmed herein by the finding that SLE patients receiving epratuzumab immunotherapy had significantly reduced CD19 compared with treatment-naïve patients.
Introduction
B-cell–directed therapy with epratuzumab has indicated clinical activity in patients with non-Hodgkin lymphoma (NHL),1,2 pediatric acute lymphoblastic leukemia,3 primary Sjögren syndrome,4 and systemic lupus erythematosus (SLE).5-7 However, its mechanism of action (MOA) remains incompletely understood. Because epratuzumab has modest antibody-dependent cellular cytotoxicity (ADCC) and negligible complement-dependent cytotoxicity when evaluated in vitro,8,9 we postulate that its therapeutic action may not result from depletion of circulating B cells, which is moderate (35% on average) in SLE patients.5 Therefore, we investigated whether ligation of epratuzumab to CD22 affects other surface molecules on B cells, focusing on modulators of B-cell antigen receptor (BCR) signaling, which may lead to altered B-cell functions that ultimately mitigate symptoms of the underlying autoimmune diseases.10,11
Trogocytosis,12 also referred to as shaving,13 is a mechanism of intercellular communication14-17 where 2 different types of cells initially form an immunologic synapse due to the interaction of receptors and ligands on acceptor and donor cells, respectively,18-20 after which, the ligands and portions of the associated donor cell membrane are taken up and subsequently internalized by the acceptor cell. Importantly, trogocytosis may regulate immune responsiveness to disease-associated antigens and can either stimulate or suppress the immune response.18
Trogocytosis of antibody-opsonized target/donor cells can be mediated by any FcγR type on various effector/acceptor cells.21-23 The effects of trogocytosis on antibody responsiveness and the induction of trogocytosis by therapeutic antibodies remain minimally studied. It has been suggested that induction of trogocytosis by excess rituximab may result in removal of rituximab-CD20 complexes from tumor cell surfaces by monocytes and natural killer (NK) cells, producing antigenic modulation and rituximab-resistant tumor cells.21 Because transfer of rituximab-CD20 complexes to monocytes is mediated by FcγR, it has also been posited that polymorphisms in FcγRII and FcγRIII may affect the degree of antibody-induced “shaving” and predict responsiveness to antibody therapy.13 In this regard, blocking trogocytosis may enhance efficacy and reduce the tumor’s escape from cytotoxicity. However, the functional consequences of antibody-mediated trogocytosis to confer a therapeutic benefit have received less attention.
Here we report for the first time that epratuzumab induces a substantial reduction of CD22, along with CD19, CD21, CD79b, CD44, CD62L, β7 integrin, and CD20, on the surface of B cells in peripheral blood mononuclear cells (PBMCs) obtained from healthy donors or SLE patients, and also on human Burkitt lymphoma cell lines spiked into normal human PBMCs. The intriguing observation that only CD22, but not other surface markers, was decreased appreciably by epratuzumab in isolated NHL cells prompted us to assess the role of FcγR-bearing effector cells, with the key finding that epratuzumab effectively mediates trogocytosis of multiple surface proteins from B cells to monocytes, NK cells, and granulocytes. Analysis of SLE patient specimens supported the notion that a similar trogocytosis mechanism occurs in vivo because, in addition to CD22, the levels of CD19, CD21, and CD79b were also significantly lower on B cells of patients receiving epratuzumab compared with those of treatment-naïve SLE patients.
Methods
Preparation of blood cell fractions
Heparinized whole blood (buffy coat) from healthy donors was purchased from The Blood Center of New Jersey (East Orange, NJ). In accordance with the Declaration of Helsinki, SLE patient whole blood was obtained with informed consent from the Wallace Rheumatic Study Center under approval of the Western Institutional Review Board. PBMCs were isolated by density gradient centrifugation on UNI-SEP tubes (Novamed Ltd., Jerusalem, Israel). Isolation/depletion of T cells and monocytes from PBMCs was accomplished using magnetic-activated cell sorting separation technology (Miltenyi Biotec, Auburn, CA) with human anti-CD3 and anti-CD14 microbeads, respectively, according to the manufacturer’s recommended protocol. For isolation of granulocytes, heparinized whole blood was layered on Ficoll-Paque Premium (GE Healthcare, Waukesha, WI) and separated from mononuclear cells by density gradient centrifugation. The remaining erythrocytes in the polymorphonuclear (granulocyte) fraction were removed with red blood cell lysing solution.
Ex vivo experiments
Unless indicated differently, PBMCs (1.5 × 106 cells/mL) were treated in triplicate with 10 µg/mL epratuzumab (or other antibodies) overnight (16-18 hours) at 37°C in nontissue culture–treated 48-well plates, before analysis by flow cytometry, following the procedures described in the supplemental Methods (see the Blood Web site). For each antigen evaluated, incubation with the isotype control labetuzumab (humanized anti-CEACAM5 IgG1) resulted in fluorescence staining that was indistinguishable from untreated cells. Where indicated, Fc receptors were blocked with Human FcR Blocking Reagent following the manufacturer’s recommendations (Miltenyi). Results shown as % of control were obtained by dividing the mean fluorescence intensity (MFI) of the epratuzumab-treated cells by that of the cells treated under the same conditions with labetuzumab and multiplying the quotient by 100. The Student t test was used to evaluate statistical significance (P < .05).
Flow cytometry
Details for the flow cytometric method, including antibodies used and gating strategies, are provided in the supplemental Methods.
Preincubation of PBMCs or Daudi cells with 100 µg/mL epratuzumab at 4°C did not inhibit detection of CD22, CD19, CD21, or CD79b with anti-CD22 clone HIB22, anti-CD19 clone HIB19, anti-CD21 clone LT21, or anti-CD79b clone CD3-1, respectively (supplemental Figure 1). On the other hand, preincubation with rituximab or humanized anti-CD19 (hA19) blocked detection of CD20 and CD19 with anti-CD20 clone LT20 or anti-CD19 clone LT19, respectively.
Fluorescence microscopy
Monocytes were purified from freshly isolated PBMCs by positive selection, and their plasma membranes were labeled with the PKH26-Red fluorescent cell labeling kit (Sigma-Aldrich, St. Louis, MO), following the manufacturer’s recommended procedure. Daudi cell plasma membranes were labeled with the PKH67-Green fluorescent cell labeling kit (Sigma-Aldrich). Fluorescent-labeled monocytes and Daudi cells were mixed 1:1 (2.5 × 106/mL for each in RPMI 1640 media) and incubated at room temperature for up to 30 minutes in the presence of 10 µg/mL epratuzumab or labetuzumab.
Results
Epratuzumab induces the reduction of specific surface proteins on B cells
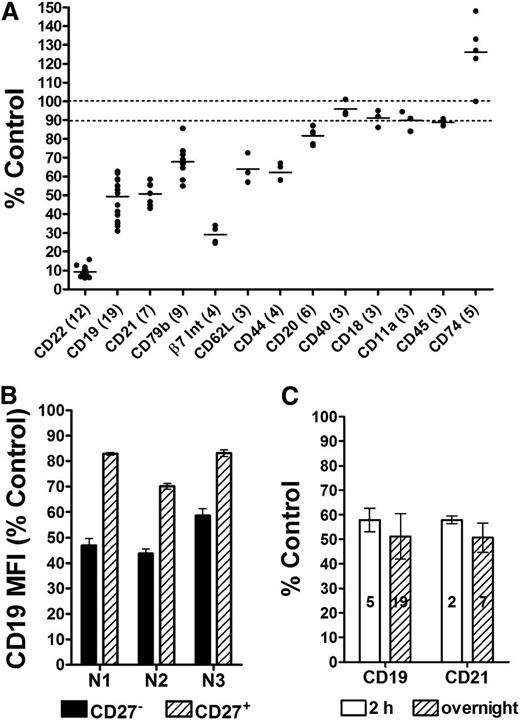
PBMCs obtained from healthy donors were incubated overnight with 10 µg/mL of either epratuzumab or an isotype control (labetuzumab), and the relative levels of various antigens on the surface of the B cells were analyzed by flow cytometry. The control antibody did not affect the levels of any of the tested antigens (supplemental Figure 2A). Based on PBMCs from 19 healthy donors assessed in various experiments, epratuzumab significantly (P < .0001) reduced the levels of the BCR modulators CD22, CD19, CD21, and CD79b to 10%, 50%, 52%, and 70%, respectively, of the level of untreated or control (Figure 1A; supplemental Figure 2B). Additionally, β7 integrin, CD62L, CD44, and CD20 were also significantly reduced (P < .0001) to 29%, 64%, 62%, and 82% of the control, respectively, whereas other surface proteins, such as CD27 (on CD27+ B cells), CD40, LFA-1 (CD18 and CD11a), and CD45 were affected minimally (<10% change) by epratuzumab. Interestingly, CD74 was the only marker tested that increased (124%; P = .0123) in response to epratuzumab; however, its lower expression as measured by MFI could inflate the observed results. Notably, CD27– naïve B cells were more responsive to epratuzumab compared with CD27+ memory B cells (Figure 1B; supplemental Figure 3; P < .0001). The effect was essentially complete within a few hours because the reductions in surface CD19 and CD21 were not significantly different following 2-hour or overnight treatment (Figure 1C).
Epratuzumab-induced reduction of select surface antigens on normal B cells. Fresh PBMCs isolated from the blood of healthy donors were treated overnight with 10 µg/mL epratuzumab or a nonbinding isotype control monoclonal antibody (mAb; labetuzumab, humanized anti-CEACAM5 IgG1κ), and the relative surface levels of selected proteins on B cells were measured using triplicate samples by flow cytometry. (A) Survey of the effect of epratuzumab on 13 different B-cell antigens. The number of donors evaluated for each specific antigen is indicated in parentheses. The levels of the key proteins were measured in 7 to 19 independent experiments. Bar represents mean values. (B) Reduction of CD19 on CD27+ and CD27– B cells. Results are from 3 independent experiments using 3 different healthy donors (N1, N2, and N3). (C) Comparison of the reduction of CD19 and CD21 on B cells following 2-hour vs overnight treatment. The number of donors evaluated for each antigen/time is indicated within the bars. Each plot is shown as the % MFI of the isotype control treatment. Each donor sample was evaluated in triplicate. Error bars represent standard deviation (SD).
Epratuzumab-induced reduction of select surface antigens on normal B cells. Fresh PBMCs isolated from the blood of healthy donors were treated overnight with 10 µg/mL epratuzumab or a nonbinding isotype control monoclonal antibody (mAb; labetuzumab, humanized anti-CEACAM5 IgG1κ), and the relative surface levels of selected proteins on B cells were measured using triplicate samples by flow cytometry. (A) Survey of the effect of epratuzumab on 13 different B-cell antigens. The number of donors evaluated for each specific antigen is indicated in parentheses. The levels of the key proteins were measured in 7 to 19 independent experiments. Bar represents mean values. (B) Reduction of CD19 on CD27+ and CD27– B cells. Results are from 3 independent experiments using 3 different healthy donors (N1, N2, and N3). (C) Comparison of the reduction of CD19 and CD21 on B cells following 2-hour vs overnight treatment. The number of donors evaluated for each antigen/time is indicated within the bars. Each plot is shown as the % MFI of the isotype control treatment. Each donor sample was evaluated in triplicate. Error bars represent standard deviation (SD).
Antibodies to CD19 and CD20 affect B cells differently from anti-CD22 mAbs
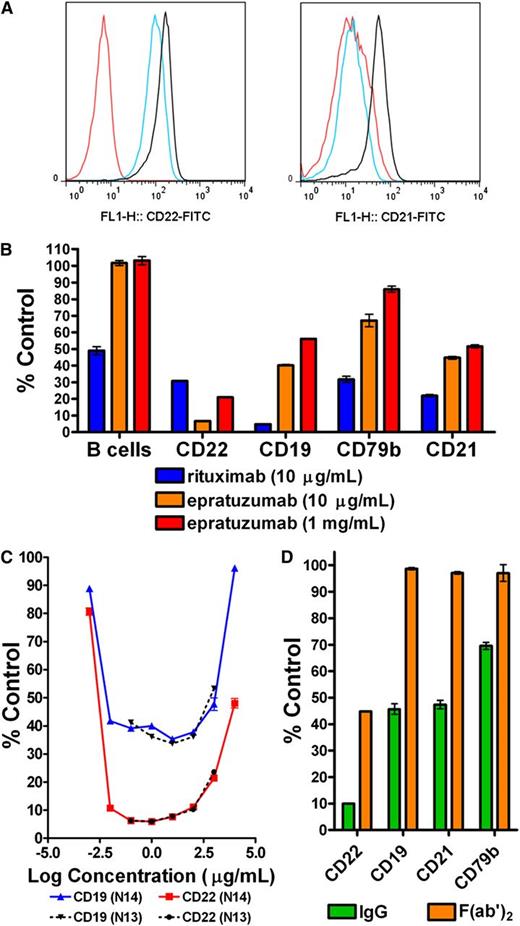
Incubation of PBMCs with a humanized version of RFB4, an anti-CD22 mAb that binds a different epitope from epratuzumab, reduced CD22, CD19, CD21, and CD79b on B cells to similar levels as epratuzumab (supplemental Figure 4). Compared with epratuzumab, hA19 moderately reduced the level of CD22 on B cells (66% of control) within PBMCs (Figure 2A). Although treatment with hA19 precluded measurement of CD19, the fact that hA19 lowered the level of CD21, to a similar level as epratuzumab, suggests that a concomitant reduction in CD19 is also likely. Rituximab diminished CD19, CD21, and CD79b to a greater extent than epratuzumab (Figure 2B), whereas CD22 was reduced to a lesser extent (supplemental Figure 5). Under the conditions examined, rituximab at 10 µg/mL reduced the B-cell count by 50%, presumably via ADCC, whereas epratuzumab did not cause B-cell depletion, either at 10 µg/mL or 1 mg/mL.
Effects of antibodies targeting CD19, CD20, or CD22. Fresh PBMCs isolated from healthy donors were treated overnight with mAbs at the indicated concentrations. (A) Histograms showing cell count vs fluorescence intensity of CD22 (left) and CD21 (right) on B cells following treatment with 10 µg/mL of epratuzumab (red), hA19 (blue), or labetuzumab (black). Representative results from 3 independent experiments, which gave similar results. (B) Relative B-cell count (B cells) and levels of CD19, CD22, CD21, and CD79b following treatment with rituximab (10 µg/mL) or epratuzumab (10 µg/mL and 1 mg/mL). A representative graph of 3 independent experiments is shown. (C) PBMCs were treated in triplicate with epratuzumab or labetuzumab at varied concentrations (1 ng/mL to 10 mg/mL). The results are shown for 2 independent experiments using 2 different normal donors (N13 and N14). (D) Cells were treated with whole IgG or a F(ab′)2 fragment of epratuzumab at 10 µg/mL. A representative graph of 3 independent experiments is shown. Each plot is shown as the % MFI of the isotype control (labetuzumab) treatment at the same protein concentration. Each sample was evaluated in triplicate. Error bars represent SD.
Effects of antibodies targeting CD19, CD20, or CD22. Fresh PBMCs isolated from healthy donors were treated overnight with mAbs at the indicated concentrations. (A) Histograms showing cell count vs fluorescence intensity of CD22 (left) and CD21 (right) on B cells following treatment with 10 µg/mL of epratuzumab (red), hA19 (blue), or labetuzumab (black). Representative results from 3 independent experiments, which gave similar results. (B) Relative B-cell count (B cells) and levels of CD19, CD22, CD21, and CD79b following treatment with rituximab (10 µg/mL) or epratuzumab (10 µg/mL and 1 mg/mL). A representative graph of 3 independent experiments is shown. (C) PBMCs were treated in triplicate with epratuzumab or labetuzumab at varied concentrations (1 ng/mL to 10 mg/mL). The results are shown for 2 independent experiments using 2 different normal donors (N13 and N14). (D) Cells were treated with whole IgG or a F(ab′)2 fragment of epratuzumab at 10 µg/mL. A representative graph of 3 independent experiments is shown. Each plot is shown as the % MFI of the isotype control (labetuzumab) treatment at the same protein concentration. Each sample was evaluated in triplicate. Error bars represent SD.
Epratuzumab reduces B-cell surface proteins over a broad concentration range
Compared with treatment at 10 µg/mL epratuzumab, incubation at 1 mg/mL resulted in significantly (P < .02) less reduction in CD22, CD19, CD79b, and CD21 (Figure 2B; supplemental Figure 6). Competition with high-concentration (1 mg/mL) labetuzumab significantly (P < .003) reduced the effect of epratuzumab (10 µg/mL) on CD22 and CD19, but to a lesser extent than high-dose epratuzumab (supplemental Figure 6). Overnight incubation of normal PBMCs with epratuzumab at concentrations ranging from 0.1 to 1000 µg/mL confirmed that doses approaching 1 mg/mL diminished reduction of CD22 (Figure 2C, donor 13). A follow-up experiment on concentrations of epratuzumab spanning 8 logs (1 ng/mL to 10 mg/mL) produced a U-shaped curve for CD22 with substantial dampening at concentrations lower than 10 ng/mL or greater than 1 mg/mL (Figure 2C, donor 14). A similar profile was observed for CD19, which was reduced to a similar level over a broad concentration range (10 ng/mL to 100 µg/mL) of epratuzumab. These results suggest that the Fc of epratuzumab might be required for its full effect on reducing the levels of these B-cell markers. Supporting this notion, epratuzumab antigen-binding fragment, F(ab′)2, moderately reduced CD22 (45% of control), as compared with the IgG (10% of control), yet had no effect on CD19, CD21, and CD79b (Figure 2D). As further support, blocking of FcγRs prior to treatment with epratuzumab significantly (P < .001) inhibited the reduction of CD22, CD19, and CD21 on B cells (supplemental Figure 7).
Epratuzumab does not reduce surface antigens other than CD22 on isolated malignant B cells (in the absence of PBMCs)
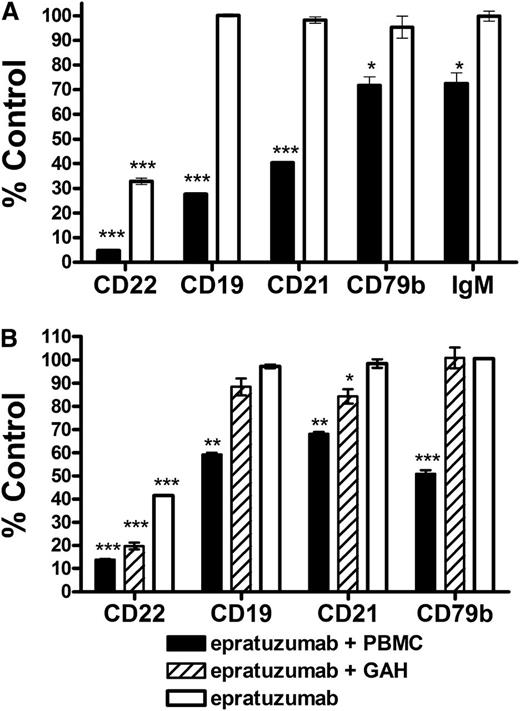
The Fc requirement was substantiated using isolated malignant B cells. In vitro, epratuzumab induced an intermediate reduction (33% of control) of CD22 on the surface of Daudi Burkitt lymphoma cells and did not affect the levels of other markers (Figure 3A). Alternatively, when Daudi cells were spiked into PBMCs, epratuzumab nearly eliminated CD22 (<5% of control) and significantly reduced CD19 (28%), CD21 (40%), CD79b (72%), and surface IgM (73%). Similar results were obtained with Raji Burkitt lymphoma cells (gating shown in supplemental Figure 8), where CD19, CD21, and CD79b were diminished by epratuzumab only in the presence of PBMCs (Figure 3B). In contrast, the addition of a cross-linking second antibody resulted in only a modest reduction of CD19, CD21, and CD79b.
Effect of epratuzumab on the surface levels of antigens on NHL cell lines. Daudi (A) or Raji (B) Burkitt lymphoma cells (1 × 105 cells) were treated overnight with 10 µg/mL epratuzumab or an isotype control mAb (labetuzumab) in the presence, or absence, of PBMCs (1 × 106) or goat-anti-human IgG (20 µg/mL) as a cross-linking second antibody. Repeat experiments gave reproducible results. Each plot is shown as the % MFI of the isotype control treatment. Each sample was evaluated in triplicate. Error bars represent SD. Significantly less than control treatment: ***P < .0001; **P < .001; *P < .05.
Effect of epratuzumab on the surface levels of antigens on NHL cell lines. Daudi (A) or Raji (B) Burkitt lymphoma cells (1 × 105 cells) were treated overnight with 10 µg/mL epratuzumab or an isotype control mAb (labetuzumab) in the presence, or absence, of PBMCs (1 × 106) or goat-anti-human IgG (20 µg/mL) as a cross-linking second antibody. Repeat experiments gave reproducible results. Each plot is shown as the % MFI of the isotype control treatment. Each sample was evaluated in triplicate. Error bars represent SD. Significantly less than control treatment: ***P < .0001; **P < .001; *P < .05.
Monocytes are involved in the epratuzumab-induced reduction of B-cell surface antigens
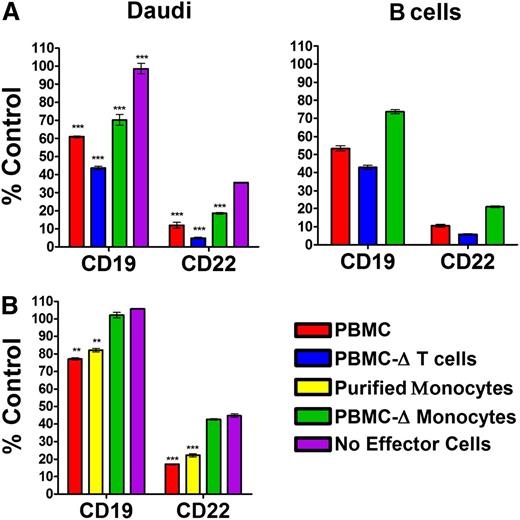
Because the surface markers were reduced only using intact IgG in the presence of PBMCs, and not with an F(ab′)2 fragment in the presence of PBMCs or with a cross-linking second antibody in place of PBMCs, we postulated the involvement of FcγR-bearing effector cells. To test this hypothesis, PBMCs were depleted of either T cells or monocytes to provide the effector cells for evaluating epratuzumab-induced reduction of CD22 and CD19 on Daudi and normal human B cells. Because the ratio of total effector cells to Daudi was held constant, removal of a specific cell type resulted in increased numbers of the remaining cell types (supplemental Figure 9). Depletion of T cells was only 50% efficient; however, this resulted in a 10% increase in monocytes and other cell types. The T-cell–depleted PBMCs were significantly (P = .005) more active than total PBMCs, indicating that T cells are not involved (Figure 4A). Indeed, purified T cells were incapable of mediating the epratuzumab-induced reduction of CD19 or CD21 on Daudi (supplemental Figure 10), apparently due to the lack of FcγRs. Conversely, depletion of monocytes significantly dampened the reduction of both CD19 and CD22 on either Daudi or normal B cells (P < .002), implicating the involvement of monocytes. That there was appreciable reduction of CD19 with the monocyte-depleted PBMCs suggests the participation of additional cell types. In a subsequent experiment, purified monocytes (supplemental Figure 11) induced a similar decrease in CD19 as the whole PBMCs, whereas the remaining monocyte-depleted PBMCs had a minimal effect, comparable to the levels measured without effector cells (Figure 4B). A similar pattern was observed for CD22.
Epratuzumab-induced reduction of CD19 and CD22 with monocytes. (A) Daudi cells (1 × 105) were mixed with effector cells (1 × 106) comprising PBMCs, T-cell–depleted PBMCs, or monocyte-depleted PBMCs, which were each derived from the same donor. (B) Daudi cells (1 × 105) were mixed with PBMCs (1 × 106), monocyte-depleted PBMCs (1 × 106), or purified monocytes (5 × 105), which were each derived from the same donor. The cell mixtures were incubated overnight with 10 µg/mL epratuzumab or an isotype control mAb (labetuzumab). The levels of CD19 and CD22 on the surface of Daudi (A, left; B) and the intrinsic B cells (A, right) were measured by flow cytometry and plotted as the % MFI of the isotype control treatment. Each sample was evaluated in triplicate. Error bars represent SD. Significantly less than treatment without effector cells: ***P < .0001; **P < .001; *P < .05.
Epratuzumab-induced reduction of CD19 and CD22 with monocytes. (A) Daudi cells (1 × 105) were mixed with effector cells (1 × 106) comprising PBMCs, T-cell–depleted PBMCs, or monocyte-depleted PBMCs, which were each derived from the same donor. (B) Daudi cells (1 × 105) were mixed with PBMCs (1 × 106), monocyte-depleted PBMCs (1 × 106), or purified monocytes (5 × 105), which were each derived from the same donor. The cell mixtures were incubated overnight with 10 µg/mL epratuzumab or an isotype control mAb (labetuzumab). The levels of CD19 and CD22 on the surface of Daudi (A, left; B) and the intrinsic B cells (A, right) were measured by flow cytometry and plotted as the % MFI of the isotype control treatment. Each sample was evaluated in triplicate. Error bars represent SD. Significantly less than treatment without effector cells: ***P < .0001; **P < .001; *P < .05.
Epratuzumab-induced reduction of B-cell antigens is a result of trogocytosis
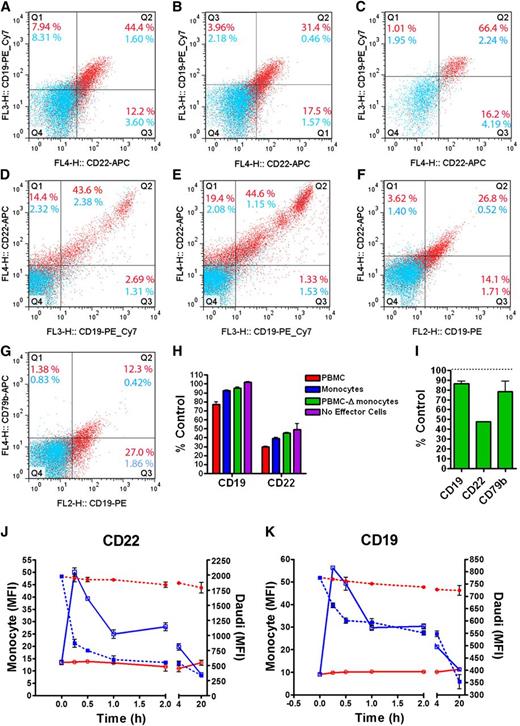
We investigated whether the process of trogocytosis occurred between target (NHL or normal B cells) and various effector cells bearing FcγRs, including CD16 and CD64. Daudi cells were mixed with PBMCs, purified monocytes, or monocyte-depleted PBMCs (supplemental Figure 12) and treated with epratuzumab for 1 hour. Daudi, monocyte, and lymphocyte populations were gated by forward (FSC) vs side scattering (SSC), which also eliminated multicell conjugates. When mixed with Daudi cells and treated with epratuzumab, purified monocytes stained positive for either CD22 (56.6% positive) or CD19 (52.4% positive), with 44% positive for both (Figure 5A). Treatment with the isotype control resulted in only 1.6% doubly positive monocytes. When the monocytes were gated further (supplemental Figures 11 and 12) into the classical CD14++CD16– (∼90%), which could mediate trogocytosis via CD64, and the proinflammatory CD14+CD16+ (∼10%) subpopulations,24 the latter exhibited 66.4% of doubly stained (CD22+CD19+) cells (Figure 5C) compared with 31.4% of the former (Figure 5B). The lymphocyte population was further gated for CD14 and CD16 to identify CD14–CD16+ NK cells (supplemental Figure 14). NK cells potently acquired CD19 and CD22 when either PBMCs (Figure 5D) or monocyte-depleted PBMCs (Figure 5E) were mixed with Daudi cells and epratuzumab. Even after only 1 hour, CD19 and CD22 were specifically reduced from Daudi cells when treated with epratuzumab in the presence of PBMCs, monocytes, or monocyte-depleted PBMCs (Figure 5H).
Trogocytosis of B-cell surface proteins to effector cells. Daudi cells were mixed with purified monocytes 1:1 (A-C), PBMCs 1:5 (D), monocyte-depleted PBMCs 1:5 (E), or purified granulocytes 1:2 (F-G) and treated for 1 hour with epratuzumab (red dots) or labetuzumab (blue dots) before analysis by flow cytometry. Monocyte, lymphocyte, granulocyte, and Daudi populations were first gated by FSC vs SSC. The monocyte gate (A) was further separated into CD14++CD16– (B) and CD14+CD16+ (C) monocyte populations, with each evaluated for CD19 and CD22 levels. CD14–CD16+ cells were isolated from the lymphocyte gate (NK cell phenotype) of complete PBMCs (D) or monocyte-depleted PBMCs (E) and evaluated for CD19 and CD22 levels. The granulocyte gate was further refined for CD16+ cells and evaluated for CD19 (F-G), CD22 (F), and CD79b (G). Representative dot plots obtained from triplicate analyses are shown. The Daudi cells (CD19+ cells in the Daudi gate) were analyzed for CD19 and CD22 levels following epratuzumab treatment with PBMCs, purified monocytes, or monocyte-depleted PBMCs (H), or purified granulocytes (I), and graphed as the % MFI of the isotype control treatment. (J-K) The levels of CD22 (J) and CD19 (K) on monocytes (solid line) and Daudi cells (dashed line) were measured over a 20-hour treatment with epratuzumab (blue line) or isotype control (red line). Each sample was evaluated in triplicate. Error bars represent SD.
Trogocytosis of B-cell surface proteins to effector cells. Daudi cells were mixed with purified monocytes 1:1 (A-C), PBMCs 1:5 (D), monocyte-depleted PBMCs 1:5 (E), or purified granulocytes 1:2 (F-G) and treated for 1 hour with epratuzumab (red dots) or labetuzumab (blue dots) before analysis by flow cytometry. Monocyte, lymphocyte, granulocyte, and Daudi populations were first gated by FSC vs SSC. The monocyte gate (A) was further separated into CD14++CD16– (B) and CD14+CD16+ (C) monocyte populations, with each evaluated for CD19 and CD22 levels. CD14–CD16+ cells were isolated from the lymphocyte gate (NK cell phenotype) of complete PBMCs (D) or monocyte-depleted PBMCs (E) and evaluated for CD19 and CD22 levels. The granulocyte gate was further refined for CD16+ cells and evaluated for CD19 (F-G), CD22 (F), and CD79b (G). Representative dot plots obtained from triplicate analyses are shown. The Daudi cells (CD19+ cells in the Daudi gate) were analyzed for CD19 and CD22 levels following epratuzumab treatment with PBMCs, purified monocytes, or monocyte-depleted PBMCs (H), or purified granulocytes (I), and graphed as the % MFI of the isotype control treatment. (J-K) The levels of CD22 (J) and CD19 (K) on monocytes (solid line) and Daudi cells (dashed line) were measured over a 20-hour treatment with epratuzumab (blue line) or isotype control (red line). Each sample was evaluated in triplicate. Error bars represent SD.
Granulocytes, or polymorphonuclear cells, which comprise mostly neutrophils, are separated from the PBMCs during processing of whole blood. Because granulocytes express FcγRIII (CD16), we evaluated their ability to participate in trogocytosis when mixed with Daudi cells and epratuzumab. Granulocytes were readily gated from the Daudi cells and multicell conjugates by FSC vs SSC and CD16 staining (supplemental Figure 15). When mixed with Daudi cells and treated with epratuzumab, but not the isotype control, 30.4%, 40.9%, and 13.7% of granulocytes stained positive for CD22, CD19, and CD79b, respectively (Figure 5F-G). Following the 1-hour incubation, a significant reduction on Daudi cells of each antigen indicates their transfer from Daudi cells to granulocytes (Figure 5I). The results of trogocytosis from the different effector cell populations are summarized in supplemental Table 1.
The events of epratuzumab-induced trogocytosis were studied by flow cytometry over 20 hours using Daudi cells and purified monocytes. Within 15 minutes, >50% of the monocytes were bound to Daudi cells due to the formation of epratuzumab-mediated immunologic synapses, and nearly half of the remaining (unconjugated) monocytes (FSClow/CD20–) were already CD22 and CD19 positive (supplemental Figure 16B). The Daudi/monocyte conjugates (CD14+CD20+CD19++FSChigh) dissociated rapidly to <5% by 4 hours (supplemental Figure 16). Although the presence of Daudi cells prohibited measurement of CD22/CD19 transfer to monocytes among the conjugates, the levels of CD22 (Figure 5J) and CD19 (Figure 5K) in the unconjugated monocytes peaked before 30 minutes and returned to baseline by 20 hours, presumably due to internalization. The levels of CD22 and CD19 on unconjugated Daudi (CD14–CD20+FSClow) decreased sharply over the first 30 minutes and then continued to decline more gradually throughout the incubation (Figure 5J-K). The isotype control did not alter the levels of either marker over the duration of the experiment.
The flow cytometry results demonstrating trogocytosis were further supported by fluorescence microscopy studies (Figure 6). Purified monocytes and Daudi cells were membrane-labeled with red and green fluorochromes, respectively, and combined. Incubation of the cell mixture with labetuzumab (isotype control) had no effect, because cells were observed predominantly (>99%) as single cells after 30 minutes (Figure 6A). Even when cells were juxtaposed, there was no evidence of synapse formation or trogocytosis (Figure 6B). Addition of epratuzumab to the cell mixture resulted in immunologic synapse formation between Daudi and monocytes within 10 minutes (Figure 6C-D), and subsequent trogocytosis of green Daudi membrane components to the red-stained monocytes (Figure 6E-I). After 30 minutes, more than 50% of each cell type was associated in various configurations including 1:1 (Figure 6F), 1 monocyte with multiple Daudi cells (Figure 6G), multiple monocytes with 1 Daudi cell (Figure 6H), and mixed cell clusters (Figure 6I).
Fluorescence microscopy showing epratuzumab-induced trogocytosis. Purified monocytes labeled with PKH26-Red fluorescence were mixed 1:1 with Daudi cells labeled with PKH67-Green fluorescence and treated with labetuzumab (A-B) or epratuzumab (C-I) at 10 µg/mL. Fluorescent images were captured with an Olympus BX66 microscope (Shinjuko, Tokyo, Japan) equipped with a Mercury-100W laser (Chiu Technical Corp., Kings Park, NY), using an Olympus ×40/0.75 air objective lens and a Kodak DC290 Camera (Rochester, NY) set at ×115 zoom. A WB filter was used to allow simultaneous fluorescence of both red and green fluorochromes. Images were captured and processed using Adobe Photoshop CS3 v10 software with a Kodak Microscopy Documentation System 290 plug-in application. Incubation times are indicated on each panel. Bars represent 5 µm. Images were obtained from 1 of 3 repeat experiments, which gave reproducible results.
Fluorescence microscopy showing epratuzumab-induced trogocytosis. Purified monocytes labeled with PKH26-Red fluorescence were mixed 1:1 with Daudi cells labeled with PKH67-Green fluorescence and treated with labetuzumab (A-B) or epratuzumab (C-I) at 10 µg/mL. Fluorescent images were captured with an Olympus BX66 microscope (Shinjuko, Tokyo, Japan) equipped with a Mercury-100W laser (Chiu Technical Corp., Kings Park, NY), using an Olympus ×40/0.75 air objective lens and a Kodak DC290 Camera (Rochester, NY) set at ×115 zoom. A WB filter was used to allow simultaneous fluorescence of both red and green fluorochromes. Images were captured and processed using Adobe Photoshop CS3 v10 software with a Kodak Microscopy Documentation System 290 plug-in application. Incubation times are indicated on each panel. Bars represent 5 µm. Images were obtained from 1 of 3 repeat experiments, which gave reproducible results.
Epratuzumab induces surface antigen reduction on B cells from treatment-naïve SLE patients
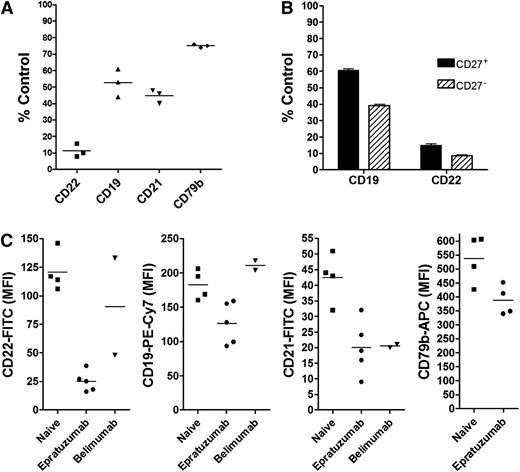
PBMCs, isolated from blood specimens of SLE patients, who had yet to receive any therapy for their disease, were treated ex vivo with epratuzumab and responded similarly to normal PBMCs, where CD22, CD19, CD21, and CD79b on the surface of B cells were reduced significantly (P < .001) to 11 ± 4%, 53 ± 8%, 45 ± 4%, and 75 ± 1% of control, respectively (Figure 7A). Also, similar to the results from normal donor PBMCs, CD27– B cells from treatment-naïve SLE patients were more responsive than CD27+ memory B cells (Figure 7B), and an F(ab′)2 fragment of epratuzumab did not induce the reduction of CD19, CD21, or CD79b (supplemental Figure 17). Interestingly, PBMCs isolated from blood specimens of SLE patients currently on epratuzumab therapy had a minimal response to the ex vivo treatment with epratuzumab (data not shown), presumably due to low levels of CD22 on their B cells as a result of therapy.
Trogocytosis of B-cell membrane proteins in SLE patients. (A) PBMCs were isolated from blood specimens of 3 treatment-naïve SLE patients and incubated overnight with 10 µg/mL epratuzumab or labetuzumab. The relative levels of CD19, CD22, CD21, and CD79b on B cells posttreatment were measured by flow cytometry. Bars represent mean values. (B) B cells were gated further into CD27+ and CD27– populations before analysis. (C) The levels of CD22, CD19, CD21, and CD79b were measured by flow cytometry on B cells gated from PBMCs that were isolated from 4 SLE patients who had yet to receive any treatment (naïve), 5 patients on active immunotherapy with epratuzumab, and 2 patients on immunotherapy with belimumab. Bars represent mean values. Each sample was evaluated in triplicate.
Trogocytosis of B-cell membrane proteins in SLE patients. (A) PBMCs were isolated from blood specimens of 3 treatment-naïve SLE patients and incubated overnight with 10 µg/mL epratuzumab or labetuzumab. The relative levels of CD19, CD22, CD21, and CD79b on B cells posttreatment were measured by flow cytometry. Bars represent mean values. (B) B cells were gated further into CD27+ and CD27– populations before analysis. (C) The levels of CD22, CD19, CD21, and CD79b were measured by flow cytometry on B cells gated from PBMCs that were isolated from 4 SLE patients who had yet to receive any treatment (naïve), 5 patients on active immunotherapy with epratuzumab, and 2 patients on immunotherapy with belimumab. Bars represent mean values. Each sample was evaluated in triplicate.
Surface antigens are reduced on B cells of SLE patients receiving epratuzumab
We further measured the relative levels of CD22, CD19, CD21, and CD79b on B cells from 5 SLE patients who were receiving epratuzumab, 4 treatment-naïve SLE patients, and 2 receiving belimumab, under identical conditions (Figure 7C). These results are summarized in supplemental Table 2 with patient profiles and treatment histories provided. Only 1 patient (S7) of the epratuzumab-treated group had a markedly reduced B-cell count; however, this patient was also taking prednisone and methotrexate. Each of the other 4 patients on epratuzumab, without methotrexate, had B-cell counts in the same range as the treatment-naïve patients. Both belimumab-treated patients had low B-cell counts. As expected, CD22 was significantly (P < .0001) lower (>80%) on the B cells of epratuzumab-treated patients (Figure 7C). Notably, CD19, CD21, and CD79b were each also significantly (P < .02) lower for the epratuzumab group (Figure 7C). Although the sample size is small, both CD19 and CD22 levels were significantly (P < .05) lower on the B cells of patients on epratuzumab compared with belimumab (Figure 7C). The level of CD21 on B cells was similarly low from the epratuzumab- and belimumab-treated patients. Because anti-CD79b-phycoerythrin (instead of allophycocyanin) was used to measure CD79b on B cells from belimumab-treated patients, we could only compare these results with 1 epratuzumab patient specimen, which was measured similarly. The CD79b-phycoerythrin MFI was greater for each of the belimumab specimens (MFI = 425 and 470) compared with that of the epratuzumab sample (MFI = 186).
Discussion
The BCR signal transduction threshold is regulated by CD19, CD22, and the CD19/CD21 complex.25-27 CD22 has been associated with both positive and negative signaling.28 In CD22-deficient mice, chronic stimulation and hyperresponses with elevated intracellular Ca2+ after BCR cross-linking were reported28-31 ; however, murine CD22−/− cells are hypoproliferative to BCR-induced stimulation, and apoptosis of naïve B cells is enhanced in CD22−/− mice.32 Alternatively, B cells from CD19-deficient mice are hyporesponsive to transmembrane signals.33-35
The present findings disclose a previously unknown, and potentially important, MOA of epratuzumab, which may be pertinent to its therapeutic effects in B-cell regulated autoimmune diseases. Based on these findings, the activity of epratuzumab on B cells is twofold, one via binding to CD22, which also occurs with F(ab′)2, and the other via engagement of FcγR-bearing effector cells. Whereas both epratuzumab and its F(ab′)2 fragment are capable of inducing internalization, as well as phosphorylation of CD22,8 with concurrent relocation to lipid rafts,36 only the former results in the trogocytosis of CD22 along with fellow BCR regulators including CD19, CD21, and CD79b, as well as the BCR itself, likely due to their close proximity to the epratuzumab-bound CD22. The pronounced (>90%) and persistent loss of CD22 on B cells by epratuzumab-mediated internalization and trogocytosis is expected to render B cells less active and less viable,32 and the accompanied decrease of CD19 could further enhance this effect. Reduction of CD19 is of particular relevance for autoimmune diseases because elevated CD19 has been correlated with susceptibility to SLE in animal models as well as in patients, and loss of CD19 should attenuate activation of B cells by raising the BCR-signaling threshold.27,31,33,34,37,38 Because CD19 is critical for mediating the initial spreading of B cells over the surface of antigen-containing membranes, CD19-deficient B cells are unable to gather sufficient antigen to trigger BCR activation.39 In addition, loss of CD19 on B cells may inhibit their activation in response to T-cell–dependent antigens.39
In agreement with Dörner and colleagues,40 adhesion molecules, including CD62L, which is involved in systemic B-cell activation, and also β7 integrin, were reduced in response to epratuzumab in the presence of PBMCs. CD44, an adhesion protein that participates in a wide variety of B-cell functions, including activation, recirculation, and homing, was also diminished with epratuzumab. We propose that epratuzumab-mediated loss of CD22 and CD19, and possibly other BCR modulators and cell-adhesion molecules, incapacitates B cells to render them unresponsive to activation by T-cell–dependent antigens, leading to therapeutic control in B-cell–mediated autoimmune diseases. Indeed, CD19, CD22, CD21, and CD79b each were significantly lower on B cells of epratuzumab-treated, compared with treatment-naïve, SLE patients, as reported here for the first time. Thus, clinical evidence supports our ex vivo results and suggests that reduction of CD19, and other key B cell proteins, via trogocytosis might be an important MOA for epratuzumab, particularly with autoimmune disease indications, where therapeutic efficacy has been demonstrated with only partial (∼35%) depletion of B cells.
Trogocytosis is not unique to epratuzumab. Both hRFB4, a humanized anti-CD22 mAb that binds to a different epitope than epratuzumab, and hA19 also mediate trogocytosis of multiple B-cell proteins, as shown here. In the present study, we confirmed the ex vivo reduction of CD19 on B cells in PBMCs by rituximab,41 which also mediated trogocytosis of CD21, CD79b, and CD22. Except for CD22, each antigen was reduced more by rituximab than epratuzumab, likely due to the ∼10-fold higher expression of CD20 compared with CD22, wherein the latter is also more rapidly internalized upon antibody ligation,8,9 and internalization is expected to compete with trogocytosis.
In chronic lymphocytic leukemia, high doses of rituximab resulted in removal of rituximab-CD20 complexes by trogocytosis to monocytes, enabling these malignant cells to escape the effects of the antibody by antigenic modulation.13 Reducing the dose limited the extent of trogocytosis and improved the therapeutic effects.42 Based on our present findings, a similar process of antigen modulation via trogocytosis induced by anti-CD22 or anti-CD20 antibodies that extends beyond the respective targeted antigens can be implicated in their therapeutic efficacy. Although the effective dose of epratuzumab (ex vivo) spans from 10 ng/mL to 100 µg/mL with little difference in the extent of trogocytosis, higher concentrations resulted in diminished trogocytosis, which could result from FcγR blocking, instead of effector-target cell cross-linking. Further, the presence of excess epratuzumab may favor monovalent antigen binding, reduced antigen clustering, and limited translocation of CD22 to lipid rafts,36 all of which could limit trogocytosis. This could also explain the clinical observations that higher doses (720 mg/m2 weekly × 4) of epratuzumab administered to SLE patients5,7 or lymphoma patients2 did not show an improvement over the midrange dose (360 mg/m2 weekly × 4) used, because the serum concentration of >150 µg/mL may reduce trogocytosis efficiency.
Clinically, an important distinction from anti-CD20 immunotherapy, which has been well documented to essentially eliminate (>90%) circulating B cells43,44 when assessed by counting CD19+ B cells, is that epratuzumab depletes only 34% to 45% of B cells6 using the same quantification method. We also found that the B-cell numbers for the specimens evaluated herein from patients currently on epratuzumab were not markedly reduced. In the overnight ex vivo assay, there was no evidence of B-cell killing induced by epratuzumab, whereas similar treatment with rituximab depleted 50% of B cells, which were identified as either CD19+ or CD22+. Our results caution that using CD19 as a marker for quantifying B cells by flow cytometry from patients treated with agents that induce CD19 trogocytosis may result in an overestimation of B-cell depletion. For example, if only CD19 gating were used, B-cell depletion by rituximab would have been measured incorrectly as 60%, and, if a tighter CD19 gating based on control treatment were used, 100% depletion would have been reported. Whereas rituximab can kill B cells by complement-dependent cytotoxicity and ADCC,45 neither of these effector functions appears to be the major MOA for epratuzumab. Although the mechanism whereby epratuzumab effects the modest depletion of B cells in vivo remains unclear, the fact that that epratuzumab therapy leaves the B-cell pool mostly intact, but likely less active, might be an advantage over CD20-directed immunotherapy of autoimmune disease.
Our results agree with other reports that trogocytosis is a fairly prevalent phenomenon by which there is an exchange of membrane molecules among immune, and also tumor, cells.21,46-51 Haas et al52 reported that treatment of mice with ligand-blocking mouse anti-mouse CD22 antibodies reduced surface levels of IgM, IgD, CD19, and CD21 on B cells. Like epratuzumab, these antibodies had minimal effects on isolated B cells, but the contrasting in vivo and in vitro results were not elaborated further. Here, we describe the trogocytosis of key BCR modulators induced with epratuzumab and propose that reduction of CD19, in addition to CD22, results in the mitigation of autoimmune diseases, including SLE, as observed in clinical trials. Whether the evaluation of such mechanisms of trogocytosis can help select other potentially effective antibodies in autoimmune disease therapy and also optimal doses remains to be investigated.
Presented in part at the 54th ASH Annual Meeting, Atlanta, GA, December 8-11, 2012; and at the 2013 EULAR Annual European Congress of Rheumatology, Madrid, Spain, June 12-15, 2013.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Jennifer Nelson for procurement of SLE blood specimens and providing patient therapy profiles.
Authorship
Contribution: E.A.R. designed the study, analyzed data, and wrote the paper; D.M.G. and C.-H.C. analyzed data and wrote the paper; R.M. and D.L.R. performed key laboratory experiments; D.J.W. provided SLE patient specimens and therapy profiles; and all authors reviewed and approved the submitted manuscript.
Conflict-of-interest disclosure: All of the authors except D.J.W. have full-time employment and or stock/options with Immunomedics, Inc. D.M.G. is an officer and director of Immunomedics, Inc.
Correspondence: Chien-Hsing Chang, Immunomedics, Inc., 300 The American Rd, Morris Plains, NJ 07950; e-mail: kchang@immunomedics.com; and David M. Goldenberg, Garden State Cancer Center, Center for Molecular Medicine and Immunology, 300 The American Rd, Morris Plains, NJ 07950; e-mail: dmg.gscancer@att.net.