In this issue of Blood, Shukla and colleagues identify IRF4−/−Vh11 mice, which develop spontaneous chronic lymphocytic leukemia (CLL) with 100% penetrance within 10 months as a novel mouse model of CLL, providing a new tool for investigating the pathogenesis of CLL and evaluating therapeutic agents.1
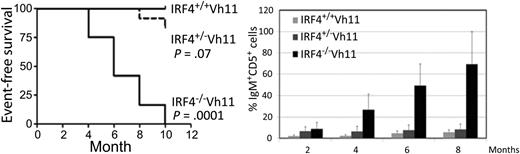
Spontaneous CLL development in IRF4−/−Vh11 mice. See Figure 1 in the article by Shukla et al that begins on page 2848.
Spontaneous CLL development in IRF4−/−Vh11 mice. See Figure 1 in the article by Shukla et al that begins on page 2848.
Genetically modified mice are essential tools for investigating the roles of genes in development and progression of diseases and for preclinical testing of new therapies. The absence of good mouse models of CLL, the most common leukemia in Western countries, has hindered research and the development of therapies to combat this incurable disease. Due to the nonproliferating nature of circulating CLL cells, xenograft models of human CLL are inaccurate models of disease. In the last decade, several mouse models representing different subtypes of CLL have been developed. For example, Eµ-TCL1 transgenic mice resemble aggressive CLL,2 Dlu2/miR15a/16-1 deletion mice3 and miR29b transgenic mice4 are related to indolent CLL, and TRAF2DN/Bcl2 double transgenic mice5 may be a model of refractory CLL. However, because of the complexity and heterogeneity of CLL and the limitations of current mouse models, the development of new mouse models of CLL is attractive. In this issue, Shukla and colleagues establish IRF4−/−Vh11 mice as a novel mouse model of CLL with 100% penetrance within 10 months.1
Interferon-regulatory factor 4 (IRF4) is a critical transcription factor for hematopoietic development and the immune system. It has been identified as an oncogene in multiple myeloma6 but a tumor suppressor in CLL.7 In this study, Shukla and colleagues backcrossed Vh11 mice which have a dramatically expanded B1 cell population into IRF4-deficiency mice and found that 100% (n = 12) of IRF4−/−Vh11 mice developed CLL within 10 months (see figure). Among those IRF4−/−Vh11 mice, 70% resemble indolent CLL and 30% exhibit aggressive CLL. Even after just 5 months, 7 of 12 (58%) IRF4−/−Vh11 mice developed CLL and the rest developed monoclonal B-cell lymphocytosis (MBL). In contrast, no CLL or MBL was developed in the IRF4+/+Vh11 control mice within 12 months. The authors also reported that IgM+CD5+ CLL cells started to increase in the blood of IRF4−/−Vh11 mice at 2 to 4 months of age and occupied ∼69% of peripheral blood mononuclear cells at 8 months of age (see figure). IRF4−/−Vh11 mice exhibited splenomegaly and lymph node enlargement and those with aggressive CLL had enlarged livers. The authors then identified the surface phenotype of IRF4−/−Vh11 CLL cells as CD19+, B220low/−, CD23−, CD21−, IgDlow, and CD1dint, and demonstrated that IRF4−/−Vh11 CLL cells were transplantable in immunodeficient host mice.
To further characterize the IRF4−/−Vh11 CLL cells, Shukla and coworkers studied proliferation, survival, and molecular signatures of these cells and found that IRF4−/−Vh11 CLL cells mainly proliferated in spleen while not in blood nor in lymph node, and these cells were resistant to apoptosis. Consistent with these findings, reexpression of IRF4 in IRF4−/−Vh11 CLL cells in vitro promoted apoptosis. Very interestingly, the authors found that the expression of Mcl-1 which is a critical prosurvival factor for CLL cells8 was significantly increased in all 5 IRF4−/−Vh11 CLL samples compared with controls, while the expression of TCL1 and miR15a/16-1 was not deregulated.
Collectively, the findings in this study1 strongly indicate an important role of IRF4 in the initiation and progression of CLL and potential applications of this novel IRF4−/−Vh11 mouse model in understanding CLL etiology and testing preclinical drugs. There are also several questions that need to be further addressed. First, in order to verify the role of IRF4 in the initiation of CLL, the transplantable ability of untransformed IRF4−/−Vh11 cells should be further investigated. Second, IRF4 deficiency affects other lymphocyte subsets9 ; therefore, other potential abnormalities in IRF4−/−Vh11 mice need to be characterized. Third, as the authors discussed in the manuscript, more generations of backcrossing are preferred to get a pure genetic background in IRF4−/−Vh11 mice to further facilitate reproducibility of experiments. Overall, this study represents a significant step forward in our understanding of CLL, and the CLL field is looking forward to the applications of this new mouse model with respect to immunology, experimental therapeutics, and biology of this disease.
Conflict-of-interest disclosure: The authors declare no competing financial interests.