In this issue of Blood, Chaturvedi and coworkers use a small molecule inhibitor of mutant isocitrate dehydrogenase 1 (IDH1) to reverse the myeloproliferative effects induced by the “oncometabolite” 2-hydroxyglutarate (2-HG).1
Mutant IDH enzymes convert a Krebs cycle intermediate, α-KG, into 2-HG. This alters the energy balance of the cell and interferes with a host of metabolic functions, including the epigenetic milieu.
Mutant IDH enzymes convert a Krebs cycle intermediate, α-KG, into 2-HG. This alters the energy balance of the cell and interferes with a host of metabolic functions, including the epigenetic milieu.
Just occasionally, things in biomedical research actually work out the way they are supposed to. A few years back, groups of researchers all around the world began using next-generation sequencing techniques to sequence whole exomes and whole genomes from acute myeloid leukemia (AML) samples, a grand scheme that was supposed to identify new mutations that could be targeted with new therapeutics. One set of mutations emerging from these screens was found in genes encoding for the 2 isoforms IDH1 and IDH2. The mutations, first identified in gliomas,2 were noted to occur in 15% to 20% of newly diagnosed AML patients, particularly in those with normal cytogenetics.3,4 After dusting off our biochemistry textbooks and reviewing the intermediates in the Krebs cycle, many of us in the field immediately wondered how such mutations could promote malignant transformation.
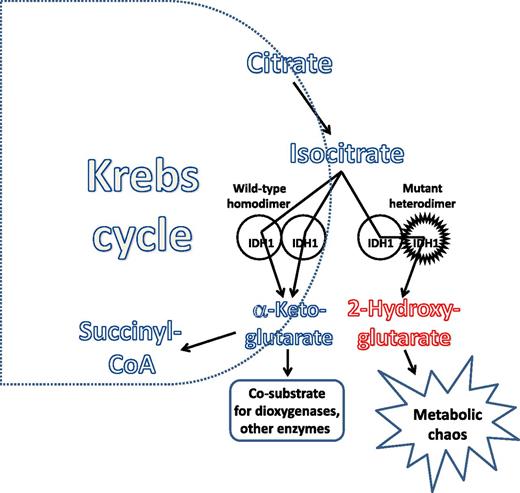
Investigators who were not intimidated by a little biochemistry took up the challenge, and a clearer picture of how these new mutations promote transformation is now emerging.5-8 The IDH enzymes, as homodimers, convert isocitrate into α-ketoglutarate (α-KG), which turns out to be not only an intermediate in the Krebs cycle, but also an essential cosubstrate for a host of cellular metabolic functions. Several different dioxygenases, including TET2 and histone demethylases (enzymes that clearly influence the epigenetic milieu of the cell), depend on α-KG for their normal function. The IDH mutations occur at critical arginine residues in the active site of the enzyme (R132 in IDH1 and R140 and R172 in IDH2). The resulting amino acid substitution not only prevents the normal catalytic function of the enzyme, it also results in a new (or neomorphic) function—the conversion of α-KG to 2-HG, which (in the case of the R-enantiomer) is a competitive inhibitor of dioxygenases. For this to work most efficiently, the mutation must occur in heterozygous form, with the wild-type enzyme generating α-KG and the mutant enzyme promptly converting it to 2-HG (see figure). The activity of the mutant heterodimer thus affects the energy landscape of the cell, and, with the generation of the 2-HG “oncometabolite,” leads to epigenetic reprogramming of a cell, blocking differentiation and contributing to a transformed phenotype. Thus, gene sequencing studies gave us the mutations, whereas the diligent work of the biochemists unraveled the mechanisms that contribute to the transformed phenotype (and, as a bonus, gave us 2 exciting new terms to bandy about: neomorphic and oncometabolite). As has been found with most other putative oncogenes in AML, IDH mutations by themselves do not appear sufficient to induce transformation.
Chaturvedi and colleagues take an important next step in this story. They and others had noted increased Hox family gene expression in association with IDH mutations. Using a retrovirally transduced murine bone marrow model, they induced a myeloproliferative phenotype when IDH1 was combined with HoxA9. The double-mutant cells contained increased levels of the inhibitory R-enantiomer of 2-HG, were cycling more, and had decreased cdk gene expression and increased mitogen-activated protein kinase activity. A small molecule inhibitor of mutant IDH1 caused decreased cell-cycling and increased apoptosis in the double-mutant cells. The same inhibitor induced apoptosis and decreased colony formation in methylcellulose of IDH1-mutant human primary bone marrow cells relative to control, thus establishing an important proof of principle that these mutant enzymes can be successfully targeted and their metabolic effects are to some degree reversible.
So, it seems that the next big target in AML has been identified. We can anticipate that the pharmaceutical–industrial complex will swing into action and launch an IDH inhibitor arms race of sorts. We can also anticipate limited single-agent activity of such drugs, given what we have learned about this disease over the past decade—namely, that AML emerges from what is at least a 2-step process and that targeting single defects (eg, as with FLT3 inhibitors) is only going to get us part way to our goal of cure. Nonetheless, we can only be encouraged by the progress we are seeing, at how chipping away at these different molecular defects will eventually cause this disease to crumble at last.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal