Key Points
UCBT is a suitable option for children with JMML, being able to cure a relevant proportion of patients.
Because disease recurrence remains the major cause of treatment failure after UCBT, strategies aimed at reducing relapse are desirable.
Abstract
We retrospectively analyzed 110 patients with juvenile myelomonocytic leukemia, given single-unit, unrelated donor umbilical cord blood transplantation. Median age at diagnosis and at transplantation was 1.4 years (age range, 0.1-6.4 years) and 2.2 years (age range, 0.5-7.4 years), respectively. Before transplantation, 88 patients received chemotherapy; splenectomy was performed in 24 patients. Monosomy of chromosome 7 was the most frequent cytogenetic abnormality, found in 24% of patients. All but 8 patients received myeloablative conditioning; cyclosporine plus steroids was the most common graft-versus-host disease prophylaxis. Sixteen percent of units were HLA-matched with the recipient, whereas 43% and 35% had either 1 or 2 to 3 HLA disparities, respectively. The median number of nucleated cells infused was 7.1 × 107/kg (range, 1.7-27.6 × 107/kg). With a median follow-up of 64 months (range, 14-174 months), the 5-year cumulative incidences of transplantation-related mortality and relapse were 22% and 33%, respectively. The 5-year disease-free survival rate was 44%. In multivariate analysis, factors predicting better disease-free survival were age younger than 1.4 years at diagnosis (hazard ratio [HR], 0.42; P = .005), 0 to 1 HLA disparities in the donor/recipient pair (HR, 0.4; P = .009), and karyotype other than monosomy 7 (HR, 0.5; P = .02). Umbilical cord blood transplantation may cure a relevant proportion of children with juvenile myelomonocytic leukemia. Because disease recurrence remains the major cause of treatment failure, strategies to reduce incidence of relapse are warranted.
Introduction
Juvenile myelomonocytic leukemia (JMML) is a unique clonal myeloproliferative disorder, typical of infancy and early childhood; it is characterized by hepatosplenomegaly and organ infiltration due to excessive proliferation of cells of the monocytic and granulocytic lineages.1 Approximately 85% of patients with JMML harbor in their leukemia cells either somatic or germ-line mutations in the genes PTPN-11, N-RAS, K-RAS, NF1, or CBL.2-6 These genetic aberrations are largely mutually exclusive, and they activate the Ras/mitogen–activated protein kinase–signaling pathway, this resulting in hypersensitivity of JMML progenitors to cytokines, specifically granulocyte-macrophage colony-stimulating factor.2 Although a small number of children with JMML, especially those with germ-line mutations of CBL and N-RAS, may experience spontaneous resolution of their myeloproliferative disorder,6,7 available evidence indicates that allogeneic hematopoietic stem cell transplantation (HSCT) remains the therapy of choice for the majority of affected children.8-13 Indeed, the median survival time of children with JMML who do not receive an allograft has been reported to be as short as 10 to 12 months.1 The clinical risk assessment in JMML includes age at diagnosis, platelet count, and percentage of fetal hemoglobin (HbF) adjusted for patient age as main prognostic variables.1,14 In particular, in large series of patients with JMML, age older than 2 years at diagnosis, a platelet count of less than 33×109/L, and levels of HbF of more than 10% are the main predictors of short survival duration.1,14
In the last 2 decades, umbilical cord blood transplantation (UCBT) from an unrelated donor (UD) has increasingly been used to treat patients with either malignant or nonmalignant diseases in need of an urgent allograft.15,16 Compared with with bone marrow transplantation (BMT), advantages of UCBT are represented by lower incidence and severity of graft-versus-host disease (GVHD), easier procurement and prompter availability of cord blood, and the possibility of using donors showing HLA disparities with the recipient.15 Several published reports have compared the outcome of UCBT and BMT from UDs in children with hematologic malignancies.17-19 In all of these studies, compared with children given BMT, recipients of UCBT received transplants from donors with greater HLA disparities, received 1-log less nucleated cells, and had delayed neutrophil and platelet recovery and lower rates of GVHD.17-19 Nevertheless, both the relapse rate (RR) and the overall survival (OS) probability did not differ between unrelated UCBT and BMT pediatric recipients.17-19 Few studies, all including a limited number of patients, have separately analyzed the outcome of children with JMML given UCBT.13,20-22 In this analysis, we report the long-term outcome of 110 children with JMML given single-unit, unmanipulated UD UCBT, and we analyze the impact of variables potentially influencing different outcomes.
Patients and methods
This study includes all patients younger than 10 years with a diagnosis of JMML, who received UCBT between 1995 and 2010 (median year, 2003) in participating institutions. Data on patient, donor, and disease characteristics, as well as on transplantation outcome, were collected by means of standardized questionnaires prepared by EUROCORD, European Working Group of Myelodysplastic Syndromes in childhood (EWOG-MDS), European Blood and Marrow Transplantation (EBMT), and by the Center for International Blood and Marrow Transplantation Research (CIBMTR) Registries for each patient enrolled into this study. EUROCORD and the CIBMTR Institutional Review Board approved the study.
In all donor-recipient pairs, histocompatibility was determined by serology tests for HLA-A and HLA-B antigens and by DNA typing for the HLA-DRB1 locus. Eighteen children (16%) received transplants with a 6/6 matched donor, 47 (43%) from a donor with a single disparity, and 39 (35%) from a donor with 2 disparities (36 patients) or 3 disparities (3 patients); data on HLA compatibility were missing in 6 patients (5.5%).
Details on patient characteristics, as well as on the median number of nucleated cells infused, are reported in Table 1. Among the 102 children for whom the information was available, 28 underwent splenectomy before transplantation. Before transplantation, 88 patients received chemotherapy; for the purpose of this analysis, patients treated with cytotoxic drugs were subdivided into 2 groups: those given acute myeloid leukemia (AML)-like combination therapy (34 children) and those receiving mild cytotoxic therapy (54 children).
Eight patients received a reduced-intensity conditioning regimen, and the remaining children were given myeloablative regimens. The most commonly used regimen, used in 49 children, consisted of busulfan, cyclophosphamide, and melphalan, according to the scheme previously reported by the EWOG-MDS group.13 Total body irradiation (TBI) was used in 19 children. GVHD prophylaxis mainly consisted of the combination of cyclosporine-A and steroids (73 patients). Serotherapy (mainly anti-thymocyte globulin) was administered before UCBT in 94 (86%) of the 110 patients analyzed. Treatment of both acute and chronic GVHD was performed according to the protocols in use at each single institution.
Usually, CB progenitors were thawed and washed before infusion, following the procedure described by Rubinstein et al.23
Definition of outcomes
OS was defined as the probability of survival, regardless of disease status, at any time point; surviving patients were censored at last follow-up, whereas only death was considered an event. Disease-free survival (DFS) was defined as the probability of being alive and disease-free at any time point; both death and relapse were considered events, and patients who were alive and disease-free were censored at last follow-up. RR was defined as the probability of having a relapse at time t, death in remission being considered the competing event. Transplantation-related mortality (TRM) was defined as the probability of dying without previous occurrence of relapse, which was considered to be the competing event. Acute and chronic GVHD were diagnosed and graded at each transplant center according to the Seattle criteria.24,25 Patients surviving for more than 14 days and those surviving for more than 100 days posttransplantation were evaluated for acute and chronic GVHD occurrence, respectively. OS, DFS, RR, TRM, and acute and chronic GVHD were estimated from the date of transplantation to the date of the statistical analysis or to the date of an adverse event. Other outcomes were (i) hematopoietic recovery: neutrophil and platelet recovery were analyzed separately, and were defined as an absolute neutrophil count (ANC) greater than 0.5 × 109/L for 3 consecutive days, and an unsupported platelet count greater than 20 × 109/L for 7 consecutive days, respectively; and (ii) graft failure was defined as either the absence of hematopoietic reconstitution of donor origin on day +60 after the allograft or second allogeneic HSCT (primary graft rejection), or as loss of donor cells after transient engraftment of donor-origin hematopoiesis. Full donor chimerism was defined as >95% of donor cells and mixed chimera between 5% and 95%. Methods of chimerism analysis varied among transplant centers.
Statistical analysis
Analysis of patient outcomes was performed on February 1, 2013. Patients were censored at the time of death or at last follow-up. Probability of OS and DFS were estimated by the Kaplan-Meier product-limit method and was expressed as percentage ± standard error. For calculation of DFS, data on patients were recorded at the time of death, relapse, or last follow-up. The probabilities of neutrophil and platelet recovery and acute and chronic GvHD were expressed as cumulative incidence (CI) curves ± standard error, using death and relapse as competing events.26,27
Univariate prognostic analyses used the log-rank test for OS or DFS, or the Gray test for other outcomes, testing the influence on each end point of patient characteristics (gender, age at diagnosis, age at transplantation, number of leukocytes at diagnosis and before UCBT, number of monocytes and platelets at diagnosis, percentage of HbF at diagnosis, blast percentage at UCBT, cytogenetic abnormalities, chemotherapy and splenectomy before UCBT, body weight, human cytomegalovirus serology testing before UCBT, ABO compatibility, interval from diagnosis to UCBT), and transplantation-related factors (number of nucleated cells infused per kilogram of body weight, number of HLA disparities, median year of transplantation, type of conditioning regimen and use of TBI, type of GVHD prophylaxis used, use of ATG). For each continuous covariate, the study population was initially split into quartiles and in 2 groups by the median. Acute GvHD was analyzed as a time-dependent covariate in a univariate model for relapse. All variables having a P value ≤ .10 in univariate analyses were included in a multivariate analysis for outcomes, which was performed using the Cox proportional regression model28 ; the proportional subdistribution hazard regression model was used to perform multivariate analyses of RI and TRM.27 All P values were 2-sided, with values of ≤ .05 indicating statistical significance. For statistical analysis, we used the SAS (SAS Inc, Cary, NC) software package.
Results
Hematopoietic recovery
Ninety children achieved neutrophil engraftment, and the median time to ANC recovery was 25 days (range 10-60). At 60 days, the CI of ANC recovery was 82% ± 4%. Platelet recovery was achieved in 76 children, the median time for obtaining a platelet count greater than 20 × 109/L being 44 days (range, 8-157 days) confidence interval; at day 180, the CI of platelet recovery was 71% ± 6%.
Information on chimerism analysis performed during the first 100 days after UCBT was available for 98 of the 110 patients. Of 81 patients reaching neutrophil recovery, 90% had full donor and 10% mixed chimera. For the remaining 17 patients, 95% had autologous recovery and 5% had transient mixed chimerism. In multivariate analysis, use of TBI during the conditioning regimen (hazard ratio [HR], 0.45; 95% confidence interval, 0.28-0.71; P < .01) and absence of monosomy 7 (HR, 0.58; 95% confidence interval, 0.34-0.93; P = .04) were associated with a greater probability of hematopoietic recovery.
Acute and chronic GVHD
Grade II to IV acute GVHD was diagnosed in 45 patients; 19 of them had grade III to IV disease. At 100 days after UCBT, the CI of grade II to IV acute GVHD was 41% ± 4%. The 92 children who were given serotherapy before the allograft had a lower CI of grade II to IV acute GVHD (38% ± 5%), compared with children who did not receive any type of serotherapy (64% ± 13%; P = .03).
Among 90 patients at risk, 16 experienced chronic GVHD, the 5-year CI being 15% ± 4%; 6 of these 16 children had the extensive form of the disease. There was a trend for a greater incidence of chronic GVHD in patients receiving transplants with units showing 2 to 3 HLA disparities (data not shown).
TRM and disease recurrence
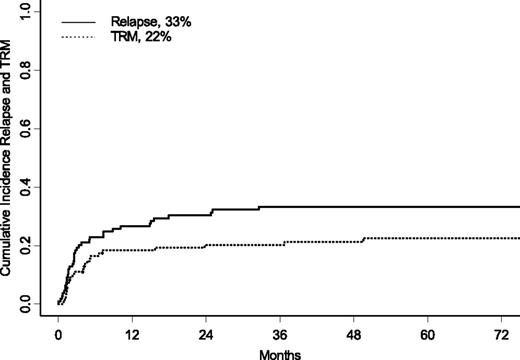
Twenty-four children died of causes attributable to transplant toxicity. Details on the causes of death are shown in Table 2. At 5 years after UCBT, the CI of TRM was 22% ± 4% (Figure 1); in univariate analysis, the presence of monosomy 7, female gender, and no cytotoxic treatment before UCBT were associated with an increased risk for TRM. The CI of TRM was 37% ± 10% for children with monosomy 7 vs 18% ± 4% for children with other karyotypes (P = .04), whereas children who were given UCBT without any previous cytotoxic treatment had a 43% ± 14% CI of TRM vs 20% ± 5% of patients treated with any type of chemotherapy before transplantation (P = .01). Girls had a CI of TRM of 26% ± 7% compared with 16% ± 5% for boys (P = .04). There was also a trend for an increased risk for TRM for patients given UCBT before 2003, the CI of TRM being 16% ± 5% for children receiving transplants after 2003 and 29% ± 6% for those given UCBT before 2003 (P = .06). In multivariate analysis, cytogenetic analysis showing monosomy 7 (HR, 3.2; P = .01), and transplantation performed before 2003 (HR, 3.7; P = .0009) were factors associated with increased TRM (see also Table 3).
Five-year CI of relapse (solid line) and TRM (dotted line) for the entire cohort of patients.
Five-year CI of relapse (solid line) and TRM (dotted line) for the entire cohort of patients.
Thirty-seven children had relapse of JMML at a median time of 2.6 months after UCBT (range, 0.33-32.5 months), and 28 of them died from disease progression after a median time of 6 months after UCBT. At 5 years, the CI of relapse was 33% ± 5% (Figure 1). Of the 9 patients who have experienced relapsed after UCBT and who remain alive, 5 received a second transplant (3 received another UCBT, and 2 received a UD HSCT); 2 were treated with chemotherapy; and for 2 patients, information on subsequent treatment is not available. In univariate analysis, children with an age at diagnosis below the median value (ie, 1.4 years old) had a lower incidence of disease recurrence (24% ± 8% vs 44% ± 8% for older children; P = .01). Use of any type of cytotoxic therapy and use of AML-like chemotherapy before UCBT were not associated with a lower risk for recurrence (data not shown). In multivariate analysis (Table 3), age at diagnosis older than 1.4 years (HR, 2.8; P = .004) remained an independent factor associated with an increased risk for relapse. A decreased incidence of relapse was associated with the presence of acute GVHD (grades II and III), when analyzed as a time-dependent covariate (P = .02). All but 1 of the 8 patients with grade IV acute GVHD died.
OS and DFS
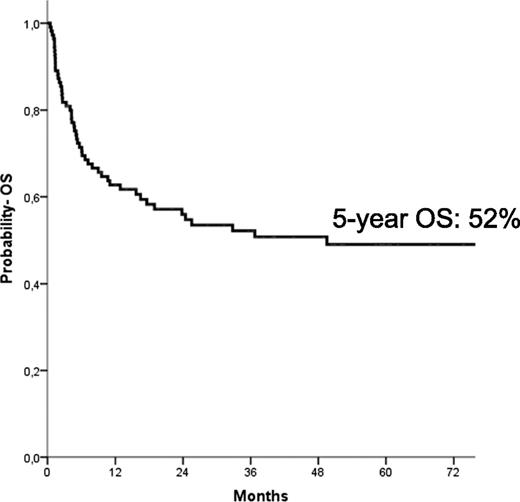
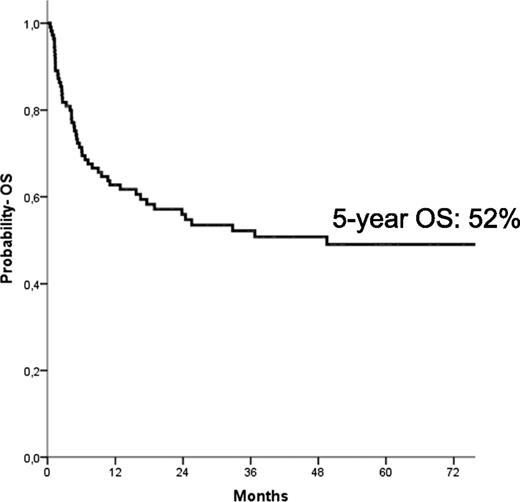
The median follow-up of the entire cohort of patients was 64 months (range, 14-174 months). At the time of the last follow-up, 58 children were alive; the Kaplan-Meier estimate of OS at 5 years was 52% ± 5% (Figure 2). In univariate analysis, children with an age at diagnosis older than 1.4 years had a lower probability of OS (42% ± 7% vs 60% ± 8% for younger children, respectively; P = .03), as well as those with monosomy 7 (30% ± 10% vs 57% ± 6% for children with other karyotypes, respectively; P = .01). Also, the use of AML-like chemotherapy before reception of the allograft was associated with a better patient outcome (53% ± 6% vs 32% ± 13% for patients who did not receive any cytotoxic drug or who received low-dose chemotherapy; P = .048). In multivariate analysis (Table 3), however, only age at diagnosis older than the median value of 1.4 years (HR, 2.3; P = .09) and the presence of monosomy 7 (HR, 2.6; P = .003) predicted a worse probability of OS.
Five-year probability of OS for the entire cohort of patients included in the analysis.
Five-year probability of OS for the entire cohort of patients included in the analysis.
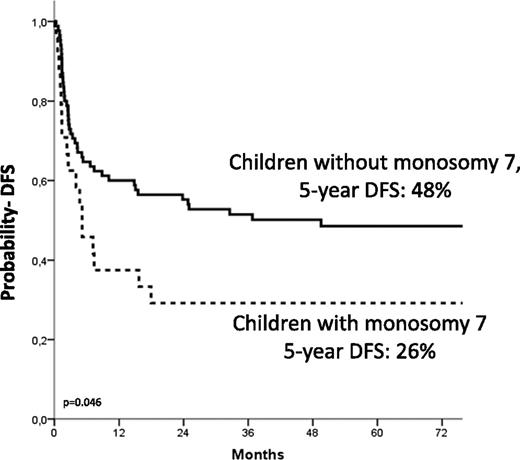
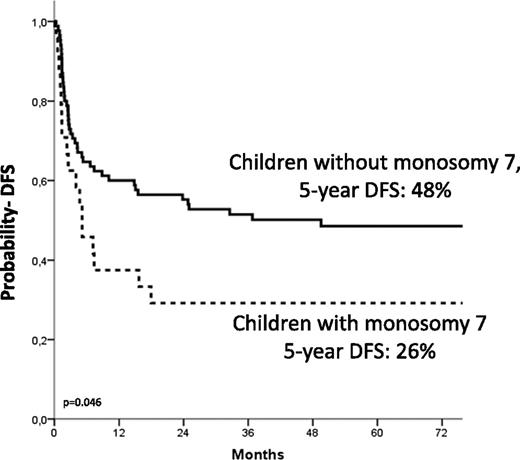
The 5-year Kaplan-Meier estimate of DFS was 44% ± 5%. Details on the correlation of the different variable analyzed with the probability of DFS are shown in Table 4. In multivariate analysis, independent factors associated with better DFS were age at diagnosis younger than 1.4 years (HR, 0.4; P = .005; Figure 3), use of donor with either 0 or 1 HLA disparity with the recipient (HR, 0.4; P = .009), and a karyotype other than monosomy 7 (HR, 0.50; P = .02; Figure 4).
Estimated 5-year probability of DFS according to age at diagnosis. Children younger than 1.4 years at diagnosis (solid line) had a better outcome compared with the older children (dotted line, P = .012).
Estimated 5-year probability of DFS according to age at diagnosis. Children younger than 1.4 years at diagnosis (solid line) had a better outcome compared with the older children (dotted line, P = .012).
Estimated 5-year probability of DFS according to the presence of monosomy of chromosome 7. Children with monosomy 7 (dotted line) had a worse outcome compared with those with other cytogenetic abnormalities or with a normal karyotype (solid line, P = .046).
Estimated 5-year probability of DFS according to the presence of monosomy of chromosome 7. Children with monosomy 7 (dotted line) had a worse outcome compared with those with other cytogenetic abnormalities or with a normal karyotype (solid line, P = .046).
Discussion
Our study reports the outcome of children with JMML given UCBT in the largest cohort ever analyzed and with a long follow-up time. Our data document that a significant proportion of children with this disease, especially when receiving transplants from donors with limited HLA disparity, can be cured with UCBT, thus indicating that this allograft can represent a suitable option for children with JMML lacking either a related donor or a UD of hematopoietic stem cells.
In the largest cohort of patients receiving HSCT for JMML published before this analysis and which included 100 patients, the 5-year probability of event-free survival for children given HSCT from either a relative or a UD was 55% and 49%, respectively.13 Similar results have been reported by other groups.10,12,29 Thus, the 5-year OS and DFS probabilities of 52% and 44%, respectively, of our UCBT recipients do not markedly differ from previously reported data on patients’ outcome when different sources or donors of hematopoietic stem cells were used.
The main advantages of use of UCBT in children with JMML refer to the observations that these children are frequently in urgent need for transplantation and that their body weight is limited. The median survival time of children with JMML without transplantation has been shown to be only 10 to 12 months.1 Thus, compared with UDs of BM and peripheral blood stem cells, the shorter interval elapsing between the start of the search for a UD and identification of a suitable cord blood unit renders UCBT an attractive option for patients with JMML.15 Many studies have demonstrated that an inverse correlation exists between the number of cord blood cells infused per kilogram of the recipient’s body weight and the risk for TRM.17-19,30 Considering that more than 90% of children with this disease are diagnosed before age 4 years, it is evident that a favorable ratio between total nucleated cells infused and recipient’s body weight can be obtained in JMML. In our UCBT recipients, the median number of cells infused was high (ie, 7.1 × 107/kg recipient body weight), being twofold greater than the number of cells before thawing recommended by the EUROCORD group (ie, at least 3.5 × 107/kg recipient body weight),31 although the range was rather large (see also Table 1 for details). Probably for this reason, we were not able to find any association of cell dose with outcomes.
Monosomy 7 and the period during which UCBT were performed correlated with an increased risk for TRM. Although we have no clear explanation for the increased risk for TRM observed in children with monosomy 7, it is noteworthy that children receiving transplants in the more recent period (ie, after 2003) had a lower risk for TRM (16%). This finding is comparable to the 13% CI of TRM after either BMT or peripheral blood transplantation13 and may be attributable to a greater awareness of the importance of factors affecting the cord blood graft choice, and, probably, to better supportive care and center experience.
Leukemia recurrence confirmed to be the most important cause of treatment failure also in this cohort of children with JMML who received UCBT. Previously published studies have reported RRs as high as 30% to 50%,10-13 values in line with the CI of relapse observed at 5 years in our children. Relapse occurs early, at a median of 2 to 4 months after transplantation and generally within the first year after the allograft. We found that children with an older age at diagnosis had an increased risk for relapse, a finding that confirms previously reported data on a detrimental effect of older patient age on the probability of survival of patients with JMML, irrespective of whether they received HSCT.1,13 The prognosis of children with JMML experiencing leukemia relapse after allogeneic HSCT remains unsatisfactory, despite the fact that salvage therapy is used. Also in our cohort, only 25% of patients experiencing relapse remain alive, mainly after having received a second allograft. Immediate withdrawal of immunosuppressive therapy for children still receiving GVHD prophylaxis has also been reported, in some cases, to lead to the eradication of the malignant cells regrowing or persisting after the conditioning regimen used in preparation for the allograft.29 It is important to note that relapse was decreased in patients presenting grade II or grade III acute GVHD (analyzed as a time-dependent covariate).
In patients with JMML, age older than 2 years, low platelet count, and high HbF at diagnosis are the main predictors of short survival duration.1,2 Age at diagnosis was also shown to significantly influence the probability of survival of patients given an allograft.10-13 We did not find any significant correlation between the platelet count at diagnosis and outcome of patients (see also Table 4), whereas the number of children in whom the information on HbF was not available (ie, n = 50) was too high to render reliable any statistical analysis on this variable. Our data confirm a worse outcome of patients diagnosed beyond ages 1 to 2 years; moreover, they indicate that patients with monosomy 7 have a lower probability of benefiting from UCBT, entirely because of an increased risk for TRM. Although a Japanese study enrolling 27 children reported a negative impact of monosomy 7,10 the most frequent cytogenetic anomaly in JMML, on the probability of OS after HSCT, other larger analyses13,31,32 documented that neither monosomy 7 nor other cytogenetic abnormalities confer a worse prognosis.
We found that children who underwent transplantation from a donor with 0 or 1 HLA disparity had a better outcome compared with those who received transplants from donors with greater HLA disparities. Our data are in line with some previously published reports showing a clear advantage for children with malignancies who underwent transplantation using HLA-matched units.19,30 However, the association of HLA disparities in the donor/recipient pair with the outcome of unrelated UCBT in patients with hematologic malignancies is controversial and is not fully established, being influenced by an interaction with the cell dose infused.
Recently, it was shown that both gene expression signatures and methylation of CpG islands in some genes affect the prognosis and the risk for recurrence after allograft reception in children with JMML.31,32 We did not have the opportunity to investigate the role of these variables, which can certainly contribute to the identification of subgroups of patients at a particularly dismal outcome, in whom, for example, epigenetic therapy and innovative strategies of immunotherapy can be tested.
It is presently controversial whether mutational status may correlate with the prognosis of children with JMML who are given HSCT. In our cohort of UCBT recipients, we could not evaluate any correlation with patient mutational status because, due to the retrospective nature of the study, data on mutations of the genes known to cause JMML were available for only 24 children.
In conclusion, our data document that UCBT can offer a chance of cure for a large proportion of children with JMML. Disease recurrence remains the major cause of treatment failure, and strategies to reduce the risk for relapse are warranted. The selection of units with a limited HLA disparity with the recipient can contribute to the improvement of a patient’s outcome.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was partially supported by grants from AIRC (Associazione Italiana Ricerca sul Cancro, progetto speciale 5xmille “INNATE IMMUNITY IN CANCER. MOLECULAR TARGETING AND CELLULAR THERAPY”), CNR (Consiglio Nazionale delle Ricerche), MIUR (Ministero dell’Istruzione, Università e della Ricerca, Progetto Rilevante Interesse Nazionale 2010), and IRCCS Ospedale Pediatrico Bambino Gesù (F.L.); a Public Health Service grant (U24-CA76518) from the National Institutes of Health National Cancer Institute; National Heart, Lung, and Blood Institute; and the National Institute of Allergy and Infectious Diseases (M.E.); and the National Institute of Health Research-Biomedical Research Centres funding scheme (V.R.)
Authorship
Contribution: F.L., M.E., E.G., and V.R. designed the study; F.L. wrote the paper; A.C., A.R., M.E., and V.R. prepared and analyzed data; J.E.W., M.L.M., M.Z., J.K., C.B., A.V., C.D.d.H., L.T., J.S., T.A.O., H.B., A.M., B.S., C.P., and C.N., provided cases for the study. All authors edited and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests..
Correspondence: Franco Locatelli, University of Pavia, IRCCS Bambino Gesù Children’s Hospital, Piazza Sant’Onofrio, 4, 00165 Rome, Italy; e-mail: franco.locatelli@opbg.net.