Key Points
Identification of a distinct leukocyte recruitment mechanism by platelet thrombi.
Leukocyte migration through thrombi is partially mediated by one or more CXCR1/2 ligands, including NAP-2.
Abstract
Thrombosis promotes leukocyte infiltration into inflamed tissues, leading to organ injury in a broad range of diseases; however, the mechanisms by which thrombi guide leukocytes to sites of vascular injury remain ill-defined. Using mouse models of endothelial injury (traumatic or ischemia reperfusion), we demonstrate a distinct process of leukocyte recruitment, termed “directed intravascular migration,” specifically mediated by platelet thrombi. Single adherent platelets and platelet aggregates stimulated leukocyte shape change at sites of endothelial injury; however, only thrombi were capable of inducing directed intravascular leukocyte migration. Leukocyte recruitment and migration induced by platelet thrombi occurred most prominently in veins but could also occur in arteries following ischemia-reperfusion injury. In vitro studies demonstrated a major role for platelet-derived NAP-2 (CXCL-7) and its CXCR1/2 receptor in regulating leukocyte polarization and motility. In vivo studies demonstrated the presence of an NAP-2 chemotactic gradient within the thrombus body. Pharmacologic blockade of CXCR1/2 as well as genetic deletion of NAP-2 markedly reduced leukocyte shape change and intrathrombus migration. These studies define a distinct process of leukocyte migration that is initiated by homotypic adhesive interactions between platelets, leading to the development of an NAP-2 chemotactic gradient within the thrombus body that guides leukocytes to sites of vascular injury.
Introduction
Ischemia-reperfusion (IR) injury is an important complication of a wide range of human diseases, including acute myocardial infarction, ischemic stroke, cardiac arrest, sickle cell crisis, and solid organ transplantation.1 A considerable body of experimental evidence has demonstrated that IR injury leads to alterations in the microcirculation and hypoperfusion of ischemic tissues.2 One of the key events promoting microvascular hypoperfusion is the development of thrombi in the microcirculation of ischemic tissue. These thrombi not only exacerbate hypoxic injury but also facilitate leukocyte recruitment to sites of organ ischemia, stimulating a potent thromboinflammatory response that further exacerbates tissue injury.3 Consistent with this, platelet depletion or inhibition of platelet activation improves microvascular perfusion and reduces tissue inflammation and injury.4-7
Experimental and clinical evidence has demonstrated that thrombi are highly efficient at recruiting leukocytes from flowing blood8 with the extent of the thromboinflammatory response correlating with the degree of organ injury and clinical outcome.9 Despite its clinical importance, there is limited understanding of the mechanisms by which microvascular thrombi guide leukocytes to sites of vascular injury. Platelet thrombi provide specific challenges for leukocyte recruitment. The three-dimensional growth of thrombi represents a physical barrier to the migration of leukocytes to sites of tissue injury. Although leukocytes have the capacity to migrate across adherent platelets in experimental chambers in vitro,10,11 it is unclear whether they migrate through or around the margins of thrombi in vivo. Furthermore, the nature of the directional cues provided by aggregated platelets that may guide leukocytes to sites of vascular injury remains unknown. Platelets are an abundant source of proinflammatory molecules that can regulate leukocyte morphology and function, including chemokines and cytokines (platelet factor 4 [PF4], neutrophil-activating peptide 2 [NAP-2], β-thromboglobulins, epithelial neutrophil activating peptide-78 [ENA-78], interleukin-8 [IL-8], regulated upon activation, normal T-cell expressed and secreted [RANTES], growth related oncogene-α, macrophage inflammatory protein-1α, and monocyte chemotactic protein-3,12 inflammatory lipids (platelet-activating factor [PAF], leukotrienes, and other arachidonic acid metabolites13 ), surface-expressed and/or shed proteins (including CD40L14 and P-selectin15 ), and microparticles (MPs). Which of these molecules plays a major role in regulating neutrophil polarization and motility, necessary for cell migration in vivo, remains unclear.
We examined leukocyte–thrombus interactions in the mesenteric circulation of mice following traumatic or IR injury and identified a distinct leukocyte recruitment mechanism mediated by platelet thrombi that is highly efficient at inducing leukocyte shape change and motility, leading to directed intravascular migration to sites of vascular injury. We demonstrated that leukocyte migration through thrombi is primarily mediated by release of CXCR1/2 ligands from platelets, predominantly the platelet α-granule chemokine NAP-2, which forms a chemotactic gradient within the thrombus body, guiding leukocytes to sites of vascular injury.
Materials and methods
Reagents
See supplemental Methods.
Mouse strains
All procedures involving mice were approved by the Alfred Medical Research and Education Precinct Animal Ethics Committee (Melbourne, Australia). GFP-mice, CD11b−/−, and P-selectin−/− were from The Jackson Laboratory (Sacramento, CA). NAP-2−/− mice generated using Velocigene technology16,17 were from the Knockout Mouse Project repository at the University of California at Davis (Davis, CA). For the targeting strategy, see supplemental Methods and supplemental Figure 1. NAP-2 deficiency was confirmed by polymerase chain reaction (supplemental Figure 2) and immunoblotting platelet lysates.
IR injury
Following a laparotomy incision, the bowel was exteriorized, and ischemia was induced by applying an arterial clip (Fine Science Tools; North Vancouver, BC, Canada) to the superior mesenteric artery for 60 minutes, and reperfusion was initiated by removing the clip as previously described.18 Sham-operated mice (mesenteric vessels exteriorized, but not clamped) served as controls. Published macroscopic and histologic grading systems were used to confirm bowel ischemia.19
Histologic quantitation of thrombosed vessels.
Following sham or IR injury, mouse small intestines were excised, fixed, and stained by using hematoxylin and eosin or Carstairs stain.20 Random sections of IR-injured or control bowel were analyzed microscopically. All vessels in the field were counted and scored according to whether they contained occlusive, partially occlusive, or no thrombi.
Intravital microscopy.
Platelet and leukocyte adhesion in mesenteric veins and bowel microvasculature was monitored via differential interference contrast (DIC) and fluorescence microscopy (IX81 invert Olympus, ×40, NA1.2), with images captured via EM-CCD camera (QuantEM 512SC; Photometrics, Tucson, AZ) and Metamorph 7.6.1 software or via confocal microscopy (Nikon A1R; Japan). Where indicated, phycoerythrin-conjugated anti–Gr-1 antibody (Ab; 100 µg/kg) or 3,3′-dihexyloxacarbocyanine iodide (100 µg/kg)/anti-GPIbβ-X649 Ab (100 µg/kg) was systemically administered to monitor leukocyte adhesion and thrombus formation, respectively.
Needle injury model
Stable thrombi were established in C57Bl/6 or GFP mouse mesenteric veins or arteries via mechanical injury alone21,22 (needle puncture using a microinjector needle tip; diameter, 2 to 4 µm; Eppendorf, Germany)], or in combination with thrombin microinjection (∼1 nL boluses of 100 U/mL, 1 to 2 cycles) ∼20 to 30 μm upstream from thrombi. Studies were performed in mesenteric veins (diameter, 120 to 180 μm) and arteries (diameter, ∼100 μm) with wall shear rates estimated to be ∼100s−1 and ∼900s−1, respectively, by using particle image velocimetry methodology.23 Platelet and leukocyte adhesion and migration were monitored using confocal or DIC and fluorescence microscopy.
Quantitative analysis of in vivo leukocyte recruitment and adhesion to thrombi.
Leukocyte rolling was defined as rolling followed by detachment, and stationary adhesion was defined as leukocyte adhesion >30 seconds. For additional information, see supplemental Methods.
Leukocyte migration.
Larger thrombi (surface area, 2500 to 10 000 µm2) were studied to quantify leukocyte migration, which was monitored at 4-second intervals for up to 30 minutes. Migrating leukocytes were defined as leukocytes that, following contact with the thrombus, underwent shape change and moved a distance of one or more cell diameters toward the site of injury.
Preparation of washed platelets and neutrophils
All procedures involving human blood collection were conducted in accordance with the Declaration of Helsinki and approved by the Monash University Standing Committee on Ethics in Research Involving Humans. Washed platelets and neutrophils were isolated as described previously.24
Preparation of platelet releasates
See supplemental Methods.
Analysis of neutrophil Mac-1 expression and activation
See supplemental Methods.
Neutrophil shape change analysis
Neutrophils (2 × 106/mL) were incubated with the indicated concentrations of platelet releasate for 20 minutes at 37°C, fixed with 2% paraformaldehyde, and examined by DIC microscopy. One unit of activity was defined as the amount of releasate required to induce shape change in 50% of the neutrophil population.
Purification of neutrophil-stimulating activity from platelet releasates
All chromatographic procedures were performed by using an Amersham-Biosciences high-pressure liquid chromatography system (Mono Q, size-exclusion and heparin-sepharose chromatographies). See supplemental Methods.
NAP-2 immunofluorescence staining of platelets
See supplemental Methods.
Characterization of NAP-2−/− mice
See supplemental Methods.
Statistical analysis
Statistical analysis was performed by using Prism Software (GraphPad; La Jolla, CA). Data are presented as means (± standard error of the mean) where n equals the number of independent experiments. See supplemental Methods.
Results
Platelet thrombi induce leukocyte recruitment and directed intravascular migration following intestinal IR injury
To investigate whether microvascular thrombi play a role in guiding leukocytes to sites of endothelial damage, we induced IR injury on a segment of mouse small intestine and examined for leukocyte–thrombus interactions in the microvasculature. Histologic analysis demonstrated that ∼25% of the intestinal microvasculature contained partially or fully occlusive thrombi, whereas no thrombi were apparent in sham-operated mice (Figure 1A-B). Microvascular thrombi contained platelets, fibrin, and leukocytes within the thrombus body (Figure 1B-C).
Platelet thrombi induce leukocyte recruitment and intravascular migration following intestinal IR injury. Spontaneous thrombus formation and leukocyte interactions in the mesenteric vasculature were examined after IR injury by using histology and real-time DIC, epifluorescence, and confocal microscopy. (A) The percentage of microvascular vessels with either partially or fully occlusive thrombi was quantified by using Carstairs staining of histologic sections (mean ± standard error of the mean [SEM]; Control group: n = 3 mice and 24 sections with 380 vessels counted; IR group: n = 5 mice and 40 histologic sections with 893 vessels counted). (B-C) Representative histologic sections of the small bowel vasculature demonstrating (B) occlusive fibrin-rich thrombi (dark red) and thrombi containing both platelets (*) and (C) fibrin with numerous leukocytes within the thrombus body after IR injury. (D) Representative DIC and fluorescence images illustrating polarized leukocytes (Gr-1 Ab, green) within the spontaneous thrombus body (DIC, demarcated) in an IR-injured mesenteric vein. (E) Representative DIC image and corresponding three-dimensional reconstruction of spontaneous platelet rich thrombi (GPIbβ Ab, blue) within the bowel wall microvasculature associated with leukocyte accumulation (Gr-1 Ab, red) after IR injury. (F) Representative images depicting polarized and spread leukocytes (Gr-1 Ab, green) in the presence [ii) Platelets] but not absence [i) Endothelium] of adherent platelets on the endothelium after IR injury. (G) The number of polarized/spread leukocytes per square millimeter on the surface of endothelium or spontaneous platelet thrombi following IR injury. *P < .05;***P < .001.
Platelet thrombi induce leukocyte recruitment and intravascular migration following intestinal IR injury. Spontaneous thrombus formation and leukocyte interactions in the mesenteric vasculature were examined after IR injury by using histology and real-time DIC, epifluorescence, and confocal microscopy. (A) The percentage of microvascular vessels with either partially or fully occlusive thrombi was quantified by using Carstairs staining of histologic sections (mean ± standard error of the mean [SEM]; Control group: n = 3 mice and 24 sections with 380 vessels counted; IR group: n = 5 mice and 40 histologic sections with 893 vessels counted). (B-C) Representative histologic sections of the small bowel vasculature demonstrating (B) occlusive fibrin-rich thrombi (dark red) and thrombi containing both platelets (*) and (C) fibrin with numerous leukocytes within the thrombus body after IR injury. (D) Representative DIC and fluorescence images illustrating polarized leukocytes (Gr-1 Ab, green) within the spontaneous thrombus body (DIC, demarcated) in an IR-injured mesenteric vein. (E) Representative DIC image and corresponding three-dimensional reconstruction of spontaneous platelet rich thrombi (GPIbβ Ab, blue) within the bowel wall microvasculature associated with leukocyte accumulation (Gr-1 Ab, red) after IR injury. (F) Representative images depicting polarized and spread leukocytes (Gr-1 Ab, green) in the presence [ii) Platelets] but not absence [i) Endothelium] of adherent platelets on the endothelium after IR injury. (G) The number of polarized/spread leukocytes per square millimeter on the surface of endothelium or spontaneous platelet thrombi following IR injury. *P < .05;***P < .001.
To investigate whether leukocytes were physically trapped or actively migrated into the thrombus, real-time intravital microscopy was performed on mesenteric veins and venules after IR injury as well as on the small intestinal microvasculature. These studies demonstrated that Gr-1–positive leukocytes (primarily neutrophils) rapidly adhered to the margins of platelet thrombi, and then developed a polarized flattened morphology and migrated into the thrombus body (Figure 1D). Furthermore, real-time confocal microcopy studies revealed extensive thrombi containing Gr-1–positive leukocytes within the intestinal microvasculature (Figure 1E and Video 1). Notably, aggregated platelets were at least 20-fold more effective at inducing leukocyte shape change and migration compared with leukocytes directly interacting with the endothelium (Figure 1F-G). In contrast, single platelets adherent to endothelial cells, although highly effective at inducing leukocyte shape change and motility, stimulated random leukocyte movement without specific directionality. These findings indicate that platelet thrombi are highly efficient at inducing directed intravascular leukocyte migration (chemotaxis) following IR injury, whereas single endothelial-bound platelets primarily stimulate leukocyte chemokinesis.
Platelet thrombi induce directed intravascular leukocyte migration at sites of traumatic endothelial injury
To investigate the mechanisms by which platelet thrombi guide leukocytes to sites of vascular injury, we used a localized endothelial injury model with microinjector needles21,22 that leads to highly reproducible platelet and leukocyte adhesion responses (Video 2). Perturbation of endothelial cell function in mesenteric veins or postcapillary venules led to the rapid formation of platelet-rich thrombi that efficiently recruited leukocytes to the thrombus surface (Figure 2A). Leukocyte recruitment by platelet thrombi commenced 2 to 3 minutes after endothelial injury and increased progressively over a 30-minute period (Figure 2A-B). Control studies confirmed that leukocyte adhesion to the thrombus surface was dependent on P-selectin (supplemental Figure 3A-B), whereas subsequent firm adhesion was primarily mediated by Mac-1 (supplemental Figure 3C-F).
Microvascular platelet thrombi induce directed intravascular leukocyte conveyance in response to localized endothelial injury. (A-F) GFP or C57Bl/6 mouse mesenteric veins were subjected to needle injury with local microinjection of thrombin, and the thrombus formation and leukocyte recruitment were monitored by confocal, epifluorescence, or DIC microscopy. (A) Representative DIC and fluorescence images of thrombi (red) and leukocyte recruitment (green) in mesenteric veins of C57Bl/6 mice following repetitive injury at the indicated time postinjury. (B) Time course of leukocyte recruitment to thrombi expressed as number per unit volume (mean ± SEM; n = 4), and quantified as described in “Materials and methods” and supplemental Methods, Quantitative analysis of leukocyte recruitment and adhesion to thrombi in vivo. (C) Left panel: representative image depicting migrating leukocytes (Gr-1 Ab, green) at different positions between the margin and the center (the site of vascular injury) of a thrombus (3,3′-dihexyloxacarbocyanine iodide [DiOC6], red) 10 minutes postinjury; the inset demonstrates the polarized morphology of individual migrating leukocytes. Right panel: migration paths (dotted lines) of individual leukocytes moving from the margins of the thrombus toward the site of vascular injury (thrombus core, red shaded area). (D) Migration velocity (µm/min) of individual leukocytes determined by using ImageJ software (n = 30). (E) Images depicting the presence of leukocytes (Gr-1 Ab, green) within the three-dimensional thrombus body (upper left), taken from front, back, and lateral perspectives 30 minutes postinjury. (F) Images depicting leukocyte migration (Gr-1 Ab, green) through the Top, Middle, and Base of a representative thrombus (red, 30-mm height as schematically depicted on the upper right) at the indicated time postinjury.
Microvascular platelet thrombi induce directed intravascular leukocyte conveyance in response to localized endothelial injury. (A-F) GFP or C57Bl/6 mouse mesenteric veins were subjected to needle injury with local microinjection of thrombin, and the thrombus formation and leukocyte recruitment were monitored by confocal, epifluorescence, or DIC microscopy. (A) Representative DIC and fluorescence images of thrombi (red) and leukocyte recruitment (green) in mesenteric veins of C57Bl/6 mice following repetitive injury at the indicated time postinjury. (B) Time course of leukocyte recruitment to thrombi expressed as number per unit volume (mean ± SEM; n = 4), and quantified as described in “Materials and methods” and supplemental Methods, Quantitative analysis of leukocyte recruitment and adhesion to thrombi in vivo. (C) Left panel: representative image depicting migrating leukocytes (Gr-1 Ab, green) at different positions between the margin and the center (the site of vascular injury) of a thrombus (3,3′-dihexyloxacarbocyanine iodide [DiOC6], red) 10 minutes postinjury; the inset demonstrates the polarized morphology of individual migrating leukocytes. Right panel: migration paths (dotted lines) of individual leukocytes moving from the margins of the thrombus toward the site of vascular injury (thrombus core, red shaded area). (D) Migration velocity (µm/min) of individual leukocytes determined by using ImageJ software (n = 30). (E) Images depicting the presence of leukocytes (Gr-1 Ab, green) within the three-dimensional thrombus body (upper left), taken from front, back, and lateral perspectives 30 minutes postinjury. (F) Images depicting leukocyte migration (Gr-1 Ab, green) through the Top, Middle, and Base of a representative thrombus (red, 30-mm height as schematically depicted on the upper right) at the indicated time postinjury.
Similar to our findings in the IR injury model, we found that platelet thrombi formed after needle injury were highly effective at inducing leukocyte migration (Figure 2C), with >50% of firmly adherent leukocytes at the base of thrombi developing a polarized flattened morphology and migrating inward from the margins of the thrombus toward the site of endothelial injury (Figure 2C; Video 3). The mean leukocyte migration velocity was 8.71 ± 0.56 μm/minutes (Figure 2D), which is comparable to leukocyte migration velocity across inflamed endothelium.25 Leukocyte migration occurred throughout the entire body of the thrombus (Figure 2E-F; Videos 4,5), with up to 30% of the thrombus coming into direct contact with migrating leukocytes (Figure 2F). Thrombus-dependent leukocyte migration also occurred in arteries following traumatic and IR injury, although to a much lesser extent than in veins (Figure 3). Overall, these findings demonstrate that platelet thrombi are highly efficient at directing leukocytes to sites of endothelial injury.
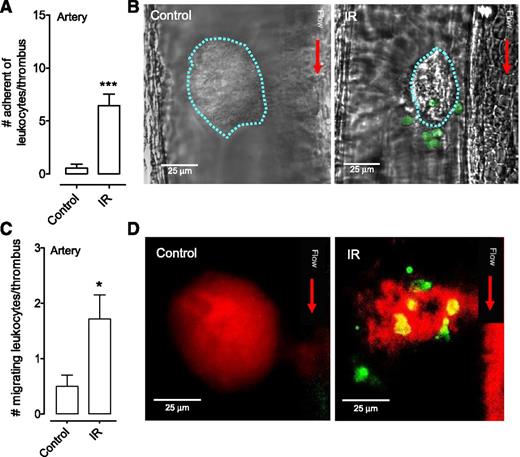
Leukocyte recruitment by platelet thrombi in injured arteries. (A-D) C57Bl/6 mice were administered an anti–Gr-1 Ab and DiOC6 prior to needle injury of arteries. Subsequent leukocyte thrombus interactions were monitored by DIC and fluorescence microscopy. The number of (A) stably adherent leukocytes and (C) migrating leukocytes to the site of vascular needle injury were quantified in sham-operated C57Bl/6 mice (Control), and mice subjected to IR injury (IR) (mean ± SEM; Control group: n = 7 mice with 13 injuries; IR group: n = 8 mice with 14 injuries). (B) Representative DIC images of thrombi induced by needle injury in sham-operated mice (Control) or mice after IR injury (IR). Note the (B) significant leukocyte recruitment (pseudo-colored green) and (D) migration (Gr-1 Ab, green) to thrombi after IR injury. *P < .05; ***P < .001.
Leukocyte recruitment by platelet thrombi in injured arteries. (A-D) C57Bl/6 mice were administered an anti–Gr-1 Ab and DiOC6 prior to needle injury of arteries. Subsequent leukocyte thrombus interactions were monitored by DIC and fluorescence microscopy. The number of (A) stably adherent leukocytes and (C) migrating leukocytes to the site of vascular needle injury were quantified in sham-operated C57Bl/6 mice (Control), and mice subjected to IR injury (IR) (mean ± SEM; Control group: n = 7 mice with 13 injuries; IR group: n = 8 mice with 14 injuries). (B) Representative DIC images of thrombi induced by needle injury in sham-operated mice (Control) or mice after IR injury (IR). Note the (B) significant leukocyte recruitment (pseudo-colored green) and (D) migration (Gr-1 Ab, green) to thrombi after IR injury. *P < .05; ***P < .001.
Platelet releasate induces neutrophil polarization
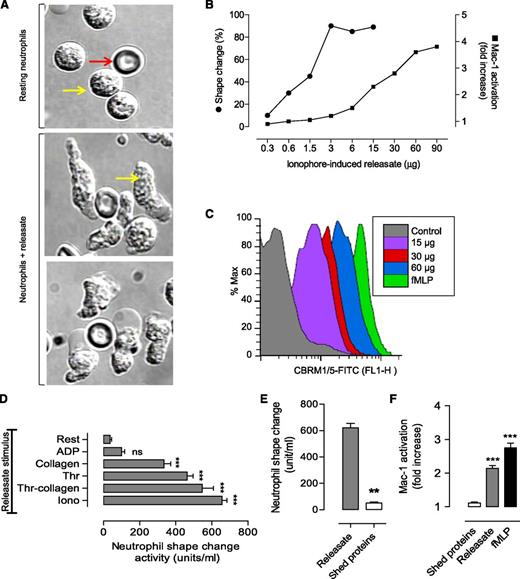
To identify the major platelet components regulating neutrophil shape change and polarization, which is necessary for cell migration, cell-free releasates from thrombin-stimulated platelets were added to isolated human neutrophils. Platelet releasates converted round neutrophils to an elongated, polarized form that had a marked increase in motility (Figure 4A). Detailed dose-response studies demonstrated that platelet releasates induced both neutrophil shape change and increased Mac-1 expression and activation, although the former was ∼20 times more sensitive to the stimulatory effects of the releasate (Figure 4B-C). Quantitative analysis revealed a good correlation between the degree of platelet α-granule release induced by different agonists (assessed by P-selectin expression in thrombin, collagen/thrombin, ionophore A23187, and collagen or adenosine diphosphate–stimulated platelets) and the total amount of neutrophil stimulating activity in releasates (Figure 4D). In contrast, neutrophil shape change and polarization did not correlate with the extent of ectodomain shedding (Figure 4E). Consistent with this, isolated shed proteins accounted for <10% of the total neutrophil-stimulating activity in platelet releasates (Figure 4F). These studies suggest that the dominant activity(s) regulating neutrophil morphologic changes are likely to be derived from platelet granules.
Platelet granule release induces neutrophil polarization and motility. Neutrophils (2 × 106/mL) were incubated with platelet releasate or shed proteins generated by the indicated agonist. Neutrophils were then assessed for shape change and Mac-1 activation, as described in “Materials and methods.” (A) Representative DIC images demonstrating neutrophil shape change following incubation with platelet releasate (29 µg/mL) for 20 minutes at 37°C (red arrow, red blood cells; yellow arrow, neutrophils). (B-C) Relative potency of platelet releasate derived from ionophore A23187-stimulated platelets: (B) line graph demonstrating the dose-dependent effects of the releasate on neutrophil shape change and Mac-1 activation, and (C) representative fluorescence-activated cell sorter (FACS) profiles of Mac-1 activation for each indicated dose of releasate (protein per milliliter of neutrophils) and formyl methionyl leucyl phenylalanine (fMLP) (2 µM). (D) Neutrophils (2 × 106/mL) were incubated in the absence (Rest) or presence of platelet releasates generated by activating platelets with either ionophore (Iono, 2 μM), thrombin + collagen (Thr-collagen, 1 U/mL thrombin and 10 μg/mL collagen), thrombin alone (Thr, 1 U/mL), collagen alone (10 μg/mL), or adenosine diphosphate (ADP; 10 μM) for 20 minutes at 37°C as described in supplemental Methods. Platelet releasate-induced neutrophil shape change was quantified as described in “Materials and methods” (mean ± SEM; n = 3). (E) The neutrophil shape change activity in platelet releasate and surface-shed proteins was quantified as described in “Materials and methods” (mean ± SEM; n = 3). (F) The effect of shed proteins and platelet releasate on Mac-1 activation was also examined, as describe in supplemental Methods and compared with that achieved in response to fMLP (2 µM) (mean ± SEM; n = 3). FITC, fluorescein isothiocyanate; ns, not significant, P > .05; **P < .01; ***P < .001.
Platelet granule release induces neutrophil polarization and motility. Neutrophils (2 × 106/mL) were incubated with platelet releasate or shed proteins generated by the indicated agonist. Neutrophils were then assessed for shape change and Mac-1 activation, as described in “Materials and methods.” (A) Representative DIC images demonstrating neutrophil shape change following incubation with platelet releasate (29 µg/mL) for 20 minutes at 37°C (red arrow, red blood cells; yellow arrow, neutrophils). (B-C) Relative potency of platelet releasate derived from ionophore A23187-stimulated platelets: (B) line graph demonstrating the dose-dependent effects of the releasate on neutrophil shape change and Mac-1 activation, and (C) representative fluorescence-activated cell sorter (FACS) profiles of Mac-1 activation for each indicated dose of releasate (protein per milliliter of neutrophils) and formyl methionyl leucyl phenylalanine (fMLP) (2 µM). (D) Neutrophils (2 × 106/mL) were incubated in the absence (Rest) or presence of platelet releasates generated by activating platelets with either ionophore (Iono, 2 μM), thrombin + collagen (Thr-collagen, 1 U/mL thrombin and 10 μg/mL collagen), thrombin alone (Thr, 1 U/mL), collagen alone (10 μg/mL), or adenosine diphosphate (ADP; 10 μM) for 20 minutes at 37°C as described in supplemental Methods. Platelet releasate-induced neutrophil shape change was quantified as described in “Materials and methods” (mean ± SEM; n = 3). (E) The neutrophil shape change activity in platelet releasate and surface-shed proteins was quantified as described in “Materials and methods” (mean ± SEM; n = 3). (F) The effect of shed proteins and platelet releasate on Mac-1 activation was also examined, as describe in supplemental Methods and compared with that achieved in response to fMLP (2 µM) (mean ± SEM; n = 3). FITC, fluorescein isothiocyanate; ns, not significant, P > .05; **P < .01; ***P < .001.
Purification, identification, and characterization of NAP-2 as the major platelet chemokine inducing neutrophil shape change and polarization
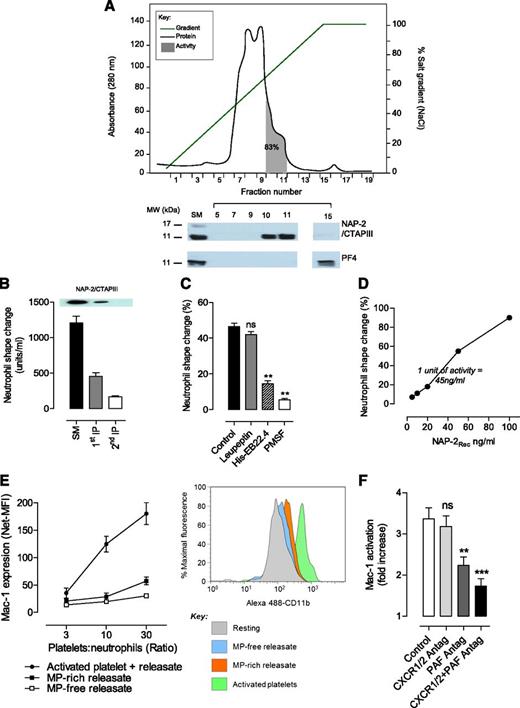
Fractionation of the platelet releasate by anion exchange chromatography revealed that ∼70% of the neutrophil-stimulating activity eluted as a single peak from anion exchange columns (supplemental Figure 4). Size-exclusion chromatography revealed the molecular weight of the active components was 20 to 30 kDa (supplemental Figure 5). Platelet-released chemokines, including connective tissue–activating peptide III (CTAP-III)/NAP-2 and PF4 tend to oligomerize forming homo- or heterodimers/trimers (20 to 30 kDa) that have high affinity for glycosaminoglycans, including heparin. Consistent with this, 83% of the total activity in platelet releasates was recovered in 2 eluting fractions from the Heparin HP Column (Figure 5A). Immunoblot analysis with anti-CTAP-III/NAP-2 or anti-PF4 monoclonal Ab revealed the presence of CTAP-III/NAP-2 in all active fractions from anion exchange (supplemental Figure 4), Heparin HP (Figure 5A), and size-exclusion columns (supplemental Figure 5). Furthermore, the quantities of CTAP-III/NAP-2 in each fraction correlated closely with the total activity-inducing neutrophil shape change and Mac-1 activation (Figure 5A; supplemental Figure 5C). PF4 was not detected in any of the peak fractions from the Heparin HP (Figure 5A) or anion exchange columns (supplemental Figure 4A-B). Consistent with this role of NAP-2, CTAP-III/NAP-2 depletion from releasates by sequential immunoprecipitation using an anti-CTAP-III/NAP-2 Ab led to an 80% loss in the total activity-inducing neutrophil shape change and polarization (Figure 5B).
Purification, identification, and characterization of NAP-2 as the major platelet-derived chemokine inducing neutrophil shape change and polarization. (A) Platelet releasate (3.42 mg, 4,000 units activity) was applied to a Heparin HP Column, bound proteins (gray line)and eluted with an NaCl gradient (0-2 M; green line); active fractions (shaded area) were identified by using the neutrophil shape change assay. Eighty-three percent of the original activity was recovered in two fractions co-eluted with NAP-2 as demonstrated by immunoblot analysis using anti–NAP-2/CTAP-III and anti-PF4 Abs. (B) Platelet releasate was subjected to 2 rounds of NAP-2 immunodepletion by using the anti–NAP-2/CTAP-III Ab, as detailed in supplemental Methods. The activity in the starting material (SM) and NAP-2–depleted releasate (first and second IP) was assessed as described above (mean ± SEM; n = 3; P < .01). Samples were also subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blotting analysis for NAP-2 (inset). (C) Neutrophils (2 × 106/mL) were first treated for 10 minutes with buffer (Control), serine protease inhibitor leupeptin (10 μg/mL), His-EB22.4 (10 μg/mL), or phenylmethanesulfonylfluoride (PMSF; 1 mM), and then incubated with platelet releasate (15 μg/mL) for 20 minutes at 37°C. The percentage of cells undergoing shape change was analyzed by DIC microscopy (percentage of total cells per field; mean ± SEM; n = 3). (D) Dose-dependent effects of recombinant NAP-2 (NAP-2Rec) on neutrophil shape change. (E-F) Regulation of Mac-1 activation through platelet–leukocyte cross-talk. (E) Relative potency of intact activated platelets (activated platelet + releaseate) versus MP-free releasate or MP-rich platelet releasate at inducing increased Mac-1 expression on the surface of isolated neutrophils. Intact activated platelets, MP-rich releasates, and MP-free releasates were prepared as described in supplemental Methods, equalized for volume, and incubated with washed neutrophils (2 × 106/mL) at the indicated platelet:neutrophil ratios for 30 minutes at 37°C. Mac-1 expression was assessed by FACS using an anti-human CD11b (ICRF44) Ab (mean ± SEM; n = 3). Representative FACS histogram profiles of Mac-1 expression at a platelet:neutrophil ratio of 30:1 are depicted on the right. (F) Neutrophils (2 × 106/mL) were treated with dimethylsulfoxide (Control), CXCR1/2 antagonist MSGA 8-73 (5 μM; CXCR1/2 Antag), or PAF antagonist CV-3988 (10 μM; PAF Antag) for 10 minutes at 37°C. The effect of PAF and CXCR1/2 antagonists on activated platelets induced Mac-1 activation and was assessed by using the CBRM1/5-FITC Ab (platelet:neutrophil ratio of 10:1) (mean ± SEM; n = 3). ns, not significant, P > .05; **P < .01; ***P < .001.
Purification, identification, and characterization of NAP-2 as the major platelet-derived chemokine inducing neutrophil shape change and polarization. (A) Platelet releasate (3.42 mg, 4,000 units activity) was applied to a Heparin HP Column, bound proteins (gray line)and eluted with an NaCl gradient (0-2 M; green line); active fractions (shaded area) were identified by using the neutrophil shape change assay. Eighty-three percent of the original activity was recovered in two fractions co-eluted with NAP-2 as demonstrated by immunoblot analysis using anti–NAP-2/CTAP-III and anti-PF4 Abs. (B) Platelet releasate was subjected to 2 rounds of NAP-2 immunodepletion by using the anti–NAP-2/CTAP-III Ab, as detailed in supplemental Methods. The activity in the starting material (SM) and NAP-2–depleted releasate (first and second IP) was assessed as described above (mean ± SEM; n = 3; P < .01). Samples were also subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blotting analysis for NAP-2 (inset). (C) Neutrophils (2 × 106/mL) were first treated for 10 minutes with buffer (Control), serine protease inhibitor leupeptin (10 μg/mL), His-EB22.4 (10 μg/mL), or phenylmethanesulfonylfluoride (PMSF; 1 mM), and then incubated with platelet releasate (15 μg/mL) for 20 minutes at 37°C. The percentage of cells undergoing shape change was analyzed by DIC microscopy (percentage of total cells per field; mean ± SEM; n = 3). (D) Dose-dependent effects of recombinant NAP-2 (NAP-2Rec) on neutrophil shape change. (E-F) Regulation of Mac-1 activation through platelet–leukocyte cross-talk. (E) Relative potency of intact activated platelets (activated platelet + releaseate) versus MP-free releasate or MP-rich platelet releasate at inducing increased Mac-1 expression on the surface of isolated neutrophils. Intact activated platelets, MP-rich releasates, and MP-free releasates were prepared as described in supplemental Methods, equalized for volume, and incubated with washed neutrophils (2 × 106/mL) at the indicated platelet:neutrophil ratios for 30 minutes at 37°C. Mac-1 expression was assessed by FACS using an anti-human CD11b (ICRF44) Ab (mean ± SEM; n = 3). Representative FACS histogram profiles of Mac-1 expression at a platelet:neutrophil ratio of 30:1 are depicted on the right. (F) Neutrophils (2 × 106/mL) were treated with dimethylsulfoxide (Control), CXCR1/2 antagonist MSGA 8-73 (5 μM; CXCR1/2 Antag), or PAF antagonist CV-3988 (10 μM; PAF Antag) for 10 minutes at 37°C. The effect of PAF and CXCR1/2 antagonists on activated platelets induced Mac-1 activation and was assessed by using the CBRM1/5-FITC Ab (platelet:neutrophil ratio of 10:1) (mean ± SEM; n = 3). ns, not significant, P > .05; **P < .01; ***P < .001.
NAP-2 is secreted by platelets as the inactive precursor CTAP-III that is converted to active NAP-2 through limited proteolysis by neutrophil membrane–associated serine proteases, principally cathepsin G.26 To confirm that proteolytic conversion of CTAP-III to active NAP-2 was relevant to neutrophil shape change, neutrophils were treated with the serine protease inhibitors phenylmethanesulfonylfluoride or His-EB22.4 (specific cathepsin G inhibitor)27 prior to incubation with platelet releasate. Both phenylmethanesulfonylfluoride and His-EB22.4 inhibited releasate-induced neutrophil shape change and polarization (Figure 5C). Furthermore, recombinant NAP-2 (NAP-2Rec) induced similar neutrophil morphologic changes over the same dose range as the purified protein (Figure 5D). Taken together, these in vitro studies demonstrated that NAP-2 is the major platelet chemokine regulating neutrophil shape change and polarization.
Platelets regulate leukocyte activation through granule release and intercellular cross-talk
In addition to releasing cytokines and chemokines, adhesion of platelets to neutrophils is associated with transcellular metabolism of eicosanoids leading to local generation of proinflammatory lipids, such as PAF, leukotrienes, and other arachidonic acid metabolites.24,28 Previous studies have defined a major role for PAF in regulating firm neutrophil adhesion to immobilized spread platelets.29 To investigate the contribution of the platelet–neutrophil adhesion response to Mac-1 activation, isolated human neutrophils were incubated with agonist-stimulated platelets, and the increase in Mac-1 expression was compared with that induced by platelet releasates. Platelet adhesion to neutrophils was associated with a dose-dependent increase in Mac-1 expression (Figure 5E). Notably, at each platelet concentration used in these assays, the increase in Mac-1 expression was much greater with intact activated platelets than with MP-free or MP-rich releasates (Figure 5E). Consistent with this, an antagonist of the NAP-2 receptors on neutrophils, CXCR1 and CXCR2 (CXCR1/2) had minimal effect on adhesion-dependent Mac-1 activation, whereas PAF receptor antagonists reduced Mac-1 expression by ∼55% and ∼70% when combined with the CXCR1/2 antagonist (Figure 5F). These findings suggest a dominant role for PAF in platelet adhesion-dependent activation of neutrophil Mac-1.
PAF and CXCR1/2 chemokines promote leukocyte recruitment and migration to sites of endothelial injury
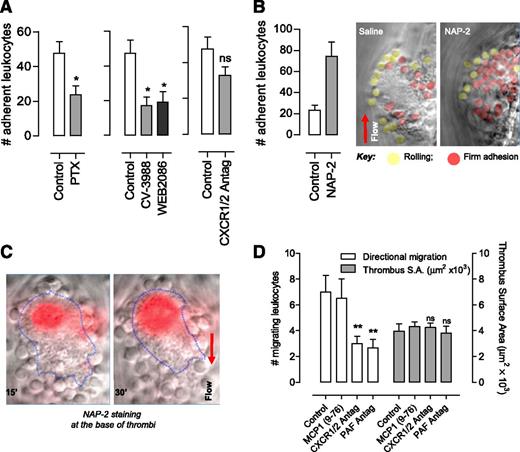
To examine the role of PAF and NAP-2 in regulating leukocyte–thrombus interactions and migration in vivo, mice were injected with a PAF receptor antagonist (CV-3988 or WEB2086), a CXCR1/2 antagonist (MSGA 8-73), or a Gαi inhibitor (pertussis toxin) prior to microvascular needle injury. Control studies confirmed that these inhibitors had no significant impact on thrombus formation or on the ability of thrombi to support leukocyte rolling or transient adhesion (supplemental Figure 6). However, pertussis toxin reduced stable leukocyte adhesion by ∼50% and PAF receptor antagonists by ∼60%, whereas the CXCR1/2 antagonist had a nonsignificant inhibitory effect on leukocyte stable adhesion (Figure 6A). This limited effect of the CXCR1/2 antagonist was not due to an inability of NAP-2 to stimulate mouse leukocyte adhesion, because microinjected recombinant NAP-2 increased firm leukocyte adhesion more than threefold (Figure 6B). Immunofluorescence analysis with an anti–NAP-2 Ab demonstrated maximal NAP-2 expression on platelets immediately adjacent to the site of vascular injury (Figure 6C) corresponding to the region of maximal platelet α-granule secretion (P-selectin expression). Similar findings were apparent with platelet aggregates formed in vitro (supplemental Figure 7). Analysis of leukocyte motility revealed an important role for both PAF and CXCR1/2 chemokines in promoting directed leukocyte migration to the site of vascular injury (Figure 6D). Taken together, these findings indicate complementary roles for PAF and CXCR1/2 chemokines in promoting leukocyte recruitment and migration through thrombi.
NAP-2 regulation of leukocyte recruitment and migration at sites of vascular injury. (A) C57Bl/6 (Control) mice were administered pertussis toxin (PTX; 4 µg per mouse, 2 hours prior to experiment), PAF antagonist (WEB2086 or CV-3988, 10 mg/kg), or the CXCR1/2 antagonist MSGA 8-73 (10 mg/kg, CXCR1/2 Antag) prior to needle injury of mesenteric veins. The number of firmly adherent leukocytes was quantified by using real-time DIC imaging 20 minutes postinjury (mean ± SEM; n = 3 to 4). (B) Recombinant NAP-2 (50 μg/mL) or saline (Control) were injected 20 to 30 μm upstream from the thrombus 10 minutes postinjury, and the number of firmly adherent leukocytes was quantified (mean ± SEM; n = 3). Representative DIC images following saline or NAP-2 injection are depicted (red, firmly adherent cells; yellow, rolling cells). (C) An anti–NAP-2 Ab (Alexa-546—labeled) was injected at the site of thrombus formation 15 minutes and 30 minutes postinjury. Images depict representative overlay images of NAP-2 staining (red) localized near the base of thrombi (blue outline) adjacent to the site of vascular injury. (D) The effects of PAF antagonist CV-3988 (PAF Antag), CXCR1/2 antagonist MSGA 8-73 (CXCR1/2 Antag), and inactive control (monocyte chemotactic protein1 9-76) on leukocyte directional migration at the thrombus base were examined over a 30-minute time frame by fluorescence microscopy (mean ± SEM; n = 3 to 4). The surface area of thrombi was also quantified. ns, not significant, P > .05; **P < .01.
NAP-2 regulation of leukocyte recruitment and migration at sites of vascular injury. (A) C57Bl/6 (Control) mice were administered pertussis toxin (PTX; 4 µg per mouse, 2 hours prior to experiment), PAF antagonist (WEB2086 or CV-3988, 10 mg/kg), or the CXCR1/2 antagonist MSGA 8-73 (10 mg/kg, CXCR1/2 Antag) prior to needle injury of mesenteric veins. The number of firmly adherent leukocytes was quantified by using real-time DIC imaging 20 minutes postinjury (mean ± SEM; n = 3 to 4). (B) Recombinant NAP-2 (50 μg/mL) or saline (Control) were injected 20 to 30 μm upstream from the thrombus 10 minutes postinjury, and the number of firmly adherent leukocytes was quantified (mean ± SEM; n = 3). Representative DIC images following saline or NAP-2 injection are depicted (red, firmly adherent cells; yellow, rolling cells). (C) An anti–NAP-2 Ab (Alexa-546—labeled) was injected at the site of thrombus formation 15 minutes and 30 minutes postinjury. Images depict representative overlay images of NAP-2 staining (red) localized near the base of thrombi (blue outline) adjacent to the site of vascular injury. (D) The effects of PAF antagonist CV-3988 (PAF Antag), CXCR1/2 antagonist MSGA 8-73 (CXCR1/2 Antag), and inactive control (monocyte chemotactic protein1 9-76) on leukocyte directional migration at the thrombus base were examined over a 30-minute time frame by fluorescence microscopy (mean ± SEM; n = 3 to 4). The surface area of thrombi was also quantified. ns, not significant, P > .05; **P < .01.
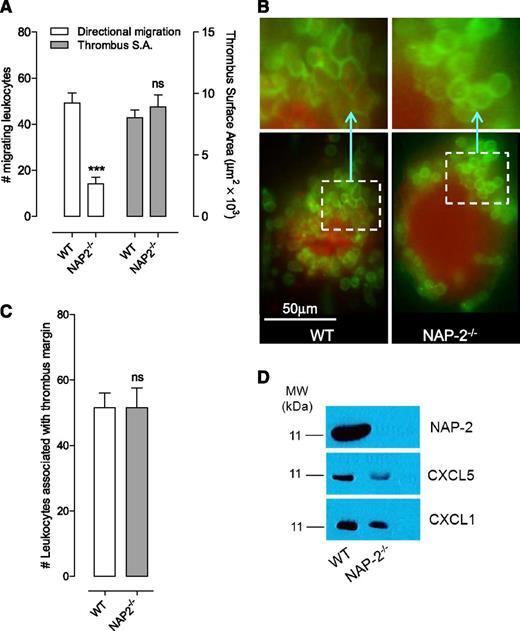
Genetic deletion of NAP-2 attenuates leukocyte migration through platelet thrombi
To investigate the importance of NAP-2 (the most abundant CXCR1/2 ligand in platelets30 ) in promoting leukocyte migration through thrombi, we used an NAP-2−/− mouse model. The phenotype of these mice has not previously been reported. NAP-2−/− mice were healthy, fertile, and bred at the expected Mendelian ratio. They exhibited normal full blood counts, and flow cytometric analysis of platelet receptor levels and activation markers were normal (supplemental Figure 8). No hemostatic defect was apparent following surgical intervention. However, intravital studies revealed a major reduction in leukocyte shape change and ∼70% decrease in leukocyte migration through thrombi to the site of vascular injury in NAP-2−/− mice relative to wild-type controls (Figure 7A-B). In control studies, we confirmed a normal platelet thrombotic response in NAP-2−/− mice (Figure 7A), and the number of Gr-1–positive leukocytes rolling on the endothelium or recruited to thrombi was indistinguishable from that of control mice (Figure 7C). Western blot analysis of platelet CXCR1/2 ligands confirmed the absence of NAP-2 and a moderate reduction in expression levels of CXCL1 and CXCL5 in NAP-2−/− mice (Figure 7D). These studies confirm a central role for platelet CXCR1/2 agonists, predominantly NAP-2, in promoting leukocyte migration through thrombi.
Leukocyte migration to the site of endothelial injury is greatly attenuated in NAP-2−/− mice. (A-C) C57Bl/6 wild-type (WT) and NAP-2−/− mice were subjected to mesenteric venous needle injury with local microinjection of thrombin. Thrombus formation and leukocyte recruitment at the thrombus base were monitored for 20 minutes by using fluorescence and DIC microscopy. (A) The number of leukocytes migrating toward the site of vascular injury was quantitated in C57Bl/6 (WT) and NAP-2−/− mice (mean ± SEM; WT group, n = 15 injuries; NAP-2−/− group, n = 15 injuries). The surface area of thrombi was also quantified. (B) Representative fluorescence images depicting leukocyte–thrombus interactions (leukocytes: Gr-1 Ab, green; platelets: DiOC6, red) in C57Bl/6 (WT) and NAP-2−/− mice; the insets demonstrate the marked reduction in shape-changed and migrating leukocytes in NAP-2−/− mice. (C) The number of leukocytes interacting with the thrombus margin was quantitated 20 minutes postinjury in C57Bl/6 (WT) and NAP-2−/− mice. (D) Platelet lysates from C57Bl/6 (WT) and NAP-2−/− mice were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blotting analysis for NAP-2, CXCL1, and CXCL5, and representative blots from 1 of 3 independent experiments are shown. ns, not significant; ***P < .001.
Leukocyte migration to the site of endothelial injury is greatly attenuated in NAP-2−/− mice. (A-C) C57Bl/6 wild-type (WT) and NAP-2−/− mice were subjected to mesenteric venous needle injury with local microinjection of thrombin. Thrombus formation and leukocyte recruitment at the thrombus base were monitored for 20 minutes by using fluorescence and DIC microscopy. (A) The number of leukocytes migrating toward the site of vascular injury was quantitated in C57Bl/6 (WT) and NAP-2−/− mice (mean ± SEM; WT group, n = 15 injuries; NAP-2−/− group, n = 15 injuries). The surface area of thrombi was also quantified. (B) Representative fluorescence images depicting leukocyte–thrombus interactions (leukocytes: Gr-1 Ab, green; platelets: DiOC6, red) in C57Bl/6 (WT) and NAP-2−/− mice; the insets demonstrate the marked reduction in shape-changed and migrating leukocytes in NAP-2−/− mice. (C) The number of leukocytes interacting with the thrombus margin was quantitated 20 minutes postinjury in C57Bl/6 (WT) and NAP-2−/− mice. (D) Platelet lysates from C57Bl/6 (WT) and NAP-2−/− mice were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and western blotting analysis for NAP-2, CXCL1, and CXCL5, and representative blots from 1 of 3 independent experiments are shown. ns, not significant; ***P < .001.
Discussion
The classical leukocyte recruitment mechanism to inflamed endothelium at sites of infection or inflammation has been extensively investigated and is well defined.31 A second leukocyte recruitment mechanism that has gained increasing recognition as an important contributor to inflammation and vascular disease involves secondary capture of leukocytes by endothelial-bound platelets.3 How platelet thrombi promote leukocyte recruitment to sites of vascular injury has remained less clearly defined, due in part to the lack of suitable experimental models to investigate this phenomenon. Here we have used a localized endothelial injury model that enables detailed analysis of the mechanisms regulating leukocyte–thrombus interactions in vivo. These studies provide the first in vivo evidence that leukocytes (predominantly neutrophils) are able to migrate through the thrombus body toward the site of initial vascular injury. Our intravital studies indicate that during the process of platelet aggregation, platelets provide a chemically designated path that promotes leukocyte trafficking from the outer margins to the core of the thrombus. Central to this model is the development of a chemotactic gradient within the body of the thrombus, which is dependent on the release of one or more chemokines within the confines of the developing aggregate (ie, maximal release of granule contents at the site of vascular injury), with progressively less degranulation to the thrombus margins. We believe this represents a distinct mechanism of leukocyte guidance because, to our knowledge, there is no precedent for homotypic interactions between cells of one type (ie, platelets) generating a chemotactic gradient to guide leukocytes to sites of vascular injury.
A prominent finding with the IR injury model in this study was the efficiency by which platelets induce leukocyte shape change and motility at sites of endothelial injury in vivo. Notably, single adherent platelets induced random leukocyte crawling (chemokinesis), whereas platelet aggregates within thrombi provided directional cues that promote leukocyte migration (chemotaxis). Leukocyte recruitment and migration by thrombi was most efficient in the venous circulation, although under certain pathological conditions, such as IR injury, this process also occurred in the arteries. These observations may help explain the importance of platelets in promoting leukocyte infiltration into ischemic tissues.9,32,33
The needle injury model used in this study produces localized injury to endothelial cells that is sufficient to induce a localized and persistent thrombotic response. Although the details of this injury model will be published elsewhere, we found that needle injury does not lead to loss of endothelial cells, but rather to perturbation of their normal antithrombotic function, leading to α-thrombin generation and rapid platelet activation. Activation of coagulation and thrombin generation on the perturbed endothelial cells is a cardinal feature of the microvascular thrombotic response characteristic of IR injury.34 It is likely that the degree of activation of coagulation and α-thrombin generation has a marked impact on platelet thromboinflammatory responses.35 In addition to a recognized role as the most potent agonist for platelet activation, P-selectin expression, and chemokine release, α-thrombin has pleiotropic effects on blood and vascular cells; therefore, its ability to induce robust inflammatory responses mediated by leukocyte recruitment at sites of injury may involve multiple pathways.36 The interplay of different molecular mechanisms, likely influenced by the nature and severity of the initial insult, may explain the variable inflammatory response associated with microvascular thrombosis and tissue ischemia37 ranging from intense, as seen in trauma38 or IR injury,39 to minor, as in the thrombotic microangiopathies (thrombotic thrombocytopenic purpura and hemolytic uremic syndrome).40,41 In agreement with this concept, platelet thrombi in the microcirculation of thrombotic thrombocytopenic purpura patients, which have limited inflammatory potential, have limited α-thrombin generation and a paucity of fibrin.42,43
Although platelets have long been recognized as a rich source of cytokines and chemoattractants,12 the function of individual chemokines has remained elusive. Furthermore, it is still unclear how a chemotactic gradient of these molecules is created to provide directional guidance for leukocytes. Our findings indicate that the graded activation status of platelets within a developing thrombus—where platelets adjacent to the initiating injury exhibit maximal degranulation that progressively decreases moving toward the outer layers of the aggregate—results in a chemically designated path directing leukocyte conveyance from the outer margins to the thrombus core. The dominant CXCR1/2 ligand produced by platelets is NAP-2, which is released at micromolar levels compared with nanomolar concentrations for other CXCR1/2 ligands, growth related oncogene-α, and ENA-78.30 Platelets are the only source of NAP-2 and store large quantities of its precursor, CTAP-III,44 which upon release becomes proteolyzed to NAP-2.26 Our studies indicate that CTAP-III/NAP-2 is retained on the surface of platelets, possibility through the interaction with glycosaminoglycans.45 NAP-2 displays an unusual biphasic chemotactic profile in which, at low concentrations (half maximal effective concentration [EC50] = 0.5 nmol/L), it stimulates neutrophil migration through engagement of CXCR2, and at higher concentrations (EC50 = 90 nmol/L), it promotes migration through CXCR1.44 Thus, it is conceivable that adherent leukocytes at the thrombus margins generate low concentrations of NAP-2, which promotes migration through CXCR2. With neutrophil migration into the thrombus body and proteolytic processing of larger quantities of CTAP-III, it is possible that migration becomes more dependent on the NAP-2–CXCR1 interaction. Although our in vitro and in vivo studies support a central role for NAP-2 in regulating leukocyte migration, we can’t exclude a potential contribution of other platelet CXCR1/2 ligands in this process, because levels of both CXCL1 and CXCL5 were moderately reduced in NAP-2−/− mice. However, given the abundance of NAP-2 in platelets and its dominant role in inducing neutrophil shape change and motility in vitro, we believe it is likely that NAP-2 is the major platelet chemokine promoting directed intravascular leukocyte migration through platelet thrombi.
Our finding that NAP-2 deficiency is associated with defective leukocyte migration at sites of vessel injury may partly explain the effectiveness of CXCR1/2 antagonists in reducing inflammatory responses and tissue injury in preclinical models of IR injury. CXCR1/2 antagonists have been demonstrated to be protective in models of cerebrovascular disease,46 and intestinal,47,48 hepatic,49 and transplant-associated IR injury. The therapeutic benefit in transplant-associated IR injury was applicable to multiple organs including kidney,50 lung,51 and liver.49 Furthermore, CXCR2−/− mice were protected from cardiac IR injury.52 Whether NAP-2 deficiency will afford similar levels of organ protection remains to be established, and studies are currently underway in our laboratory to address this issue.
Our observations that other CXCL platelet chemokines are reduced in the NAP-2−/− mouse was unexpected, given the specificity of the NAP-2 targeting strategy. Interestingly, it has been demonstrated that CXCL5 deletion leads to reductions in platelet NAP-2 levels.53 In preliminary studies, we have found that PF4 levels are reduced in NAP-2−/− platelets (unpublished observations; Z.S.K. and S.P.J.). It is well known that NAP-2 heterodimerizes with other CXCL family members,54 raising the possibility that NAP-2 heterodimerization may be required for posttranscriptional events linked to intracellular protein sorting or protein degradation. Future studies will be required to address this issue. Nonetheless, our findings suggest that conclusions on the role of individual platelet chemokines in the pathogenesis of a specific disease process may need to be interpreted with some caution if they are based solely on the findings from a platelet chemokine knockout mouse. In future studies, it will be important to assess the levels of all platelet chemokines in NAP-2, ENA-78, and PF4 knockout mice and perform complementary studies with chemokine receptor antagonists and/or specific neutralizing antibodies to more clearly define the role of individual chemokines in specific disease processes.
Overall, our studies provide a mechanistic explanation for the ability of platelet thrombi to mediate efficient accumulation of leukocytes to sites of endothelial injury. Moreover, they demonstrate major differences in the mechanisms employed by platelet thrombi to recruit leukocytes to sites of vascular injury relative to the classical leukocyte recruitment mechanisms used by inflamed endothelium. Exploiting these differences pharmacologically may lead to development of new approaches that reduce proinflammatory effects of platelet thrombi while minimizing the impact on innate immune mechanisms important for host defenses and tissue repair.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Joy Yao, Stephen Cody (Monash MicroImaging), and Dr Justin Hamilton for technical assistance, and Dr Warwick Nesbit and Dr Susan Cranmer for intellectual input. The authors also thank Dr Morty Poncz for constructive input and suggestions.
This work was supported by the National Health and Medical Research Council (NHMRC) and the Australian Research Council. S.P.J. is an NHMRC Australia Fellow and Z.S.K. is an NHMRC and National Heart Foundation postgraduate scholarship holder.
Authorship
Contribution: M.G. and Z.S.K. designed and performed experiments, analyzed data, prepared figures, and co-wrote the paper; I.A. performed experiments, analyzed data, and prepared figures; S.M.S. analyzed data and prepared figures; K.J.A. performed experiments; E.W. provided technical input; E.H. analyzed data and prepared figures; H.H.S. provided intellectual input; R.S. and S.R.M. provided reagents; M.J.H. provided intellectual input; Z.M.R. co-wrote the paper; Y.Y. designed and performed experiments, analyzed data, and co-wrote the paper; and S.P.J. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shaun P. Jackson, Australian Centre for Blood Diseases, Alfred Medical Research and Education Precinct, 6th Level, Burnet Tower, 89 Commercial Rd, Melbourne, VIC 3004, Australia; e-mail: shaun.jackson@monash.edu.
References
Author notes
M.G. and Z.S.K. contributed equally to this study as first authors.
Y.Y. and S.P.J. contributed equally to this study as senior authors.

![Figure 1. Platelet thrombi induce leukocyte recruitment and intravascular migration following intestinal IR injury. Spontaneous thrombus formation and leukocyte interactions in the mesenteric vasculature were examined after IR injury by using histology and real-time DIC, epifluorescence, and confocal microscopy. (A) The percentage of microvascular vessels with either partially or fully occlusive thrombi was quantified by using Carstairs staining of histologic sections (mean ± standard error of the mean [SEM]; Control group: n = 3 mice and 24 sections with 380 vessels counted; IR group: n = 5 mice and 40 histologic sections with 893 vessels counted). (B-C) Representative histologic sections of the small bowel vasculature demonstrating (B) occlusive fibrin-rich thrombi (dark red) and thrombi containing both platelets (*) and (C) fibrin with numerous leukocytes within the thrombus body after IR injury. (D) Representative DIC and fluorescence images illustrating polarized leukocytes (Gr-1 Ab, green) within the spontaneous thrombus body (DIC, demarcated) in an IR-injured mesenteric vein. (E) Representative DIC image and corresponding three-dimensional reconstruction of spontaneous platelet rich thrombi (GPIbβ Ab, blue) within the bowel wall microvasculature associated with leukocyte accumulation (Gr-1 Ab, red) after IR injury. (F) Representative images depicting polarized and spread leukocytes (Gr-1 Ab, green) in the presence [ii) Platelets] but not absence [i) Endothelium] of adherent platelets on the endothelium after IR injury. (G) The number of polarized/spread leukocytes per square millimeter on the surface of endothelium or spontaneous platelet thrombi following IR injury. *P < .05;***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-09-459636/5/m_4555f1.jpeg?Expires=1769091928&Signature=pn3ZPSADeRI1ClMf1uuW4DMFCF4qXpE2SUR7EXe5x8CuephtfTrk2NtQwYRqFSL-oem2h38HP0bgSl78ScjOyrlnanWq6jwDtdeSJUAaSCoRCrqiv5M6-6X-BFt9-OOQcwacdZEPi0UaJQwI7LWyDyQYXnyCwzWFZaAvRlQh3~9A6gNUK7xlO2B4mCiigX9tT8IXdLLZfQeW1oZRLYNqW8Rm32oFQlCo~YZOce4mVrjGD86~kKfZhyRNdLAuMhbhf5Xk3wqoZSHMJui6cwcq-OmVIzcHpizagtciHhiXgp8k2hE7AC~Hc7RBPFYRppOBMP7zdEjoPS4qQvxLXjxcbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Microvascular platelet thrombi induce directed intravascular leukocyte conveyance in response to localized endothelial injury. (A-F) GFP or C57Bl/6 mouse mesenteric veins were subjected to needle injury with local microinjection of thrombin, and the thrombus formation and leukocyte recruitment were monitored by confocal, epifluorescence, or DIC microscopy. (A) Representative DIC and fluorescence images of thrombi (red) and leukocyte recruitment (green) in mesenteric veins of C57Bl/6 mice following repetitive injury at the indicated time postinjury. (B) Time course of leukocyte recruitment to thrombi expressed as number per unit volume (mean ± SEM; n = 4), and quantified as described in “Materials and methods” and supplemental Methods, Quantitative analysis of leukocyte recruitment and adhesion to thrombi in vivo. (C) Left panel: representative image depicting migrating leukocytes (Gr-1 Ab, green) at different positions between the margin and the center (the site of vascular injury) of a thrombus (3,3′-dihexyloxacarbocyanine iodide [DiOC6], red) 10 minutes postinjury; the inset demonstrates the polarized morphology of individual migrating leukocytes. Right panel: migration paths (dotted lines) of individual leukocytes moving from the margins of the thrombus toward the site of vascular injury (thrombus core, red shaded area). (D) Migration velocity (µm/min) of individual leukocytes determined by using ImageJ software (n = 30). (E) Images depicting the presence of leukocytes (Gr-1 Ab, green) within the three-dimensional thrombus body (upper left), taken from front, back, and lateral perspectives 30 minutes postinjury. (F) Images depicting leukocyte migration (Gr-1 Ab, green) through the Top, Middle, and Base of a representative thrombus (red, 30-mm height as schematically depicted on the upper right) at the indicated time postinjury.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-09-459636/5/m_4555f2.jpeg?Expires=1769091928&Signature=mFaVrpRAL8cufl91oigiFqMEGv62HTtUOA~4adbbn6pJ9h2Ssxu5oh7ny38dGa6tFZ1B7KjXNZ9sikgfTra1IjE5qCtlLWOzyD-84YCjtIUUKhMSxx6K9eCF5itmFYFnVCX-BA0fJk0iS1Z3NGyTeTwxufeJth1KKdg9tK-UU~hMshHYbu-jt6Hml3xsTePSNLrX7u5JQ6MhHADG5R9h3v7WPHRJjgCMbAAml8JqexUs4gG6RykbQjcwRv2Uu~n4Io6Ji6RBZXg6UIwZAymxuyRJLbcpDQj6HU3fdcH-sIX8GAuTsXcwC-xUqYZ5ZrU9HYk2WCm3nH6MlIHU9pr~tQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Platelet thrombi induce leukocyte recruitment and intravascular migration following intestinal IR injury. Spontaneous thrombus formation and leukocyte interactions in the mesenteric vasculature were examined after IR injury by using histology and real-time DIC, epifluorescence, and confocal microscopy. (A) The percentage of microvascular vessels with either partially or fully occlusive thrombi was quantified by using Carstairs staining of histologic sections (mean ± standard error of the mean [SEM]; Control group: n = 3 mice and 24 sections with 380 vessels counted; IR group: n = 5 mice and 40 histologic sections with 893 vessels counted). (B-C) Representative histologic sections of the small bowel vasculature demonstrating (B) occlusive fibrin-rich thrombi (dark red) and thrombi containing both platelets (*) and (C) fibrin with numerous leukocytes within the thrombus body after IR injury. (D) Representative DIC and fluorescence images illustrating polarized leukocytes (Gr-1 Ab, green) within the spontaneous thrombus body (DIC, demarcated) in an IR-injured mesenteric vein. (E) Representative DIC image and corresponding three-dimensional reconstruction of spontaneous platelet rich thrombi (GPIbβ Ab, blue) within the bowel wall microvasculature associated with leukocyte accumulation (Gr-1 Ab, red) after IR injury. (F) Representative images depicting polarized and spread leukocytes (Gr-1 Ab, green) in the presence [ii) Platelets] but not absence [i) Endothelium] of adherent platelets on the endothelium after IR injury. (G) The number of polarized/spread leukocytes per square millimeter on the surface of endothelium or spontaneous platelet thrombi following IR injury. *P < .05;***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-09-459636/5/m_4555f1.jpeg?Expires=1769091929&Signature=ShuuzRmtGOpU4smOS5JM2ph5O45~LToVnIQo7PZEmZJ5AYxkpQRPisMLJJcd9AcSvkmKcjFMmvP775amPfYCP2lNzmAODTB2BSCiDuA7Yr3VYc2230o8bBDSsvCpa1r5k1nK3bT521QSC6GJtG~SHHGxO7PDYHiM81ujEJ1ZjpEOipHTx8bbp-VVbDmAllw5RjCqrONDwYwouZTM0a8trcrzms0L2hDMJhGjjeL23VuSIvDS1ByoEjLbnvxzo6bYq37lVMXWfWM1xiPX5jrHH2nDmIVGep9sbtLRLdkOHEM-06Ckq5Q8SJMOwMNlx61Zw1YcrWwQTx~B4NE-STPWgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Microvascular platelet thrombi induce directed intravascular leukocyte conveyance in response to localized endothelial injury. (A-F) GFP or C57Bl/6 mouse mesenteric veins were subjected to needle injury with local microinjection of thrombin, and the thrombus formation and leukocyte recruitment were monitored by confocal, epifluorescence, or DIC microscopy. (A) Representative DIC and fluorescence images of thrombi (red) and leukocyte recruitment (green) in mesenteric veins of C57Bl/6 mice following repetitive injury at the indicated time postinjury. (B) Time course of leukocyte recruitment to thrombi expressed as number per unit volume (mean ± SEM; n = 4), and quantified as described in “Materials and methods” and supplemental Methods, Quantitative analysis of leukocyte recruitment and adhesion to thrombi in vivo. (C) Left panel: representative image depicting migrating leukocytes (Gr-1 Ab, green) at different positions between the margin and the center (the site of vascular injury) of a thrombus (3,3′-dihexyloxacarbocyanine iodide [DiOC6], red) 10 minutes postinjury; the inset demonstrates the polarized morphology of individual migrating leukocytes. Right panel: migration paths (dotted lines) of individual leukocytes moving from the margins of the thrombus toward the site of vascular injury (thrombus core, red shaded area). (D) Migration velocity (µm/min) of individual leukocytes determined by using ImageJ software (n = 30). (E) Images depicting the presence of leukocytes (Gr-1 Ab, green) within the three-dimensional thrombus body (upper left), taken from front, back, and lateral perspectives 30 minutes postinjury. (F) Images depicting leukocyte migration (Gr-1 Ab, green) through the Top, Middle, and Base of a representative thrombus (red, 30-mm height as schematically depicted on the upper right) at the indicated time postinjury.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/22/10.1182_blood-2012-09-459636/5/m_4555f2.jpeg?Expires=1769091929&Signature=uqJXouvtLgkxMCVIZ2iWE~16~kx0IKLCFyKt7JMXMsCn5d4kIZlpnTVNc4fkUqI1E1EQjjhi8DuEng5wu-HIOuz2bww0DaNNNkkSXaIySx6AmORbP1OhvAiX2SFfhig8F6mR~ZDIvJPYyJI6wRR5IRM~77EW3yMmbaj49O9tHvkS-VE4f8BBRHmnehYyj30xtgZQs8Cwgwrc07nRzj4-sJ~mznDg80SwrqMsT2zjb8MSYCeHtzZVhAvkOCKqf9HROK9m4RgxvCQA614fKV3uhVgqXyTFmVu9E0DLl05YVAnSHtULQACknKRJY0GxEZNFX3WG2fJHVtc8LqgIYlOgWA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)