Key Points
Chromosomal breaks in BCL6 translocations occur at two distinct DNA sequence motifs associated with AID activity.
Motif usage correlates with the type of BCL6 partner gene, suggesting mechanistic differences between Ig–BCL6 and non-Ig–BCL6 rearrangements.
Abstract
BCL6 translocations are common in B-cell lymphomas and frequently have chromosomal breaks in immunoglobulin heavy chain (IgH) switch regions, suggesting that they occur during class-switch recombination. We analyze 120 BCL6 translocation breakpoints clustered in a 2156-bp segment of BCL6 intron 1, including 62 breakpoints (52%) joined to IgH, 12 (10%) joined to Ig light chains, and 46 (38%) joined to non-Ig partners. The BCL6 breaks in Ig–BCL6 translocations prefer known activation-induced cytosine deaminase (AID) hotspots such as WGCW and WRC (W = A/T, R = A/G), whereas BCL6 breaks in non-Ig rearrangements occur at CpG/CGC sites in addition to WGCW. Unlike previously identified CpG breaks in pro-B/pre-B-cell translocations, the BCL6 breaks do not show evidence of recombination activating gene or terminal deoxynucleotidyl transferase activity. Both WGCW/WRC and CpG/CGC breaks at BCL6 are most likely initiated by AID in germinal center B-cells, and their differential use suggests subtle mechanistic differences between Ig–BCL6 and non-Ig–BCL6 rearrangements.
Introduction
The CCND1 and BCL2 translocations in many human lymphomas appear to arise in B-cell progenitors in the bone marrow and to depend on the recombination activating gene (RAG) endonuclease complex for chromosome breaks at the JH and DH segments in the immunoglobulin heavy chain (IgH) locus. We previously showed that the IgH partner breakpoints in these translocations target the dinucleotide sequence CpG,1,2 and we proposed a chromosome breakage model in which activation-induced cytosine deaminase (AID) creates T:G mismatches at methylated CpG sites. More recently, we showed that some mantle cell lymphomas have CCND1 breakpoints that occur near the motif WGCW (W = A or T) and that the MYC breakpoints in Burkitt lymphoma, which occur in germinal center B-cells, strongly prefer the same WGCW motif.3 We propose a model in which CpGs are targeted in translocations occurring in pro-B-cells, when AID levels are low, whereas WGCW is preferred when AID levels are higher, as in germinal center B-cells, where class-switch recombination (CSR), somatic hypermutation (SHM), and MYC translocations occur. We also found that most CpG breaks in pro-B translocations occurred at CGC, a known AID hotspot.3
BCL6 is the most commonly rearranged gene in diffuse large B-cell lymphoma, but mechanistic insight into the basis of the chromosomal breaks in BCL6 translocations has been scant (see supplemental Figure 1, available with other supplemental data on the Blood website). Here, we analyze a large set of BCL6 chromosomal translocations to the IgH switch (SH) regions, Ig light chain (IgL) loci, and non-Ig partners, and we show that these breaks target CpG and CGC motifs, in addition to the canonical AID motifs WGCW, WGC, and WRC (R = A or G). Interestingly, the BCL6 breaks that partner with Ig loci occur near the WGCW, WGC, and WRC motifs, but not at CpG, whereas those fused to non-Ig partners occur near both CpG/CGC and WGCW/WGC/WRC motifs. Our findings raise the possibility that BCL6 breaks partner with Ig and non-Ig loci using somewhat different mechanisms.
Study design
A total of 137 unpublished and published junctional sequences were used in this study.4 Statistical methods are described in Tsai et al2 and supplemental Data.
Samples were collected from Kyoto University Hospital and affiliated hospitals. Approval was obtained from the Kyoto University Institutional Review Board for the study. Informed consent was provided according to the Declaration of Helsinki.
Results and discussion
We gathered the breakpoint sequences of 137 known and previously unpublished BCL6 chromosomal translocations. We found that 120 (87.6%) of the breakpoints cluster within a 2156-bp region at the beginning of BCL6 intron 1 (Figure 1). We then analyzed these clustered breakpoints for their proximity to numerous sequence motifs.2 After separating the BCL6 breakpoints into those that partnered with Ig and non-Ig partner loci, 2 patterns emerged.
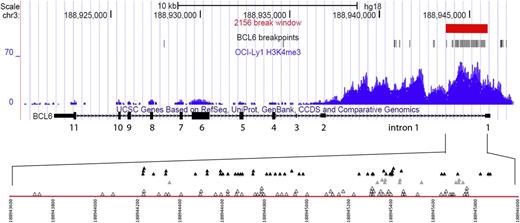
BCL6 translocation breakpoints in human B-cell lymphomas. The top 3 tracks in this University of California, Santa Cruz, Genome Browser snapshot show the 2156-bp BCL6 break window defined in this study (red), the location of BCL6 breaks analyzed here (black), and the H3K4me3 chromatin immunoprecipitation–seq read density in the lymphoma cell line OCI-Ly1 (blue). The fourth track shows exons 1 to 11 of the BCL6 gene (rectangles), with exon 1 at the far right. Exons 1, 2, 3, and parts of exons 4 and 11 are untranslated. The red line in the bottom panel is a magnified view of the 2156-bp break window within BCL6 intron 1. The numbering corresponds to human chromosome 3q (NCBI36/hg 18). The open triangles denote BCL6 breaks in IgH–BCL6 translocations, the gray triangles denote BCL6 breaks in IgL translocations (11 λ and 1 κ), and the filled black triangles denote BCL6 breaks in non-Ig translocations.
BCL6 translocation breakpoints in human B-cell lymphomas. The top 3 tracks in this University of California, Santa Cruz, Genome Browser snapshot show the 2156-bp BCL6 break window defined in this study (red), the location of BCL6 breaks analyzed here (black), and the H3K4me3 chromatin immunoprecipitation–seq read density in the lymphoma cell line OCI-Ly1 (blue). The fourth track shows exons 1 to 11 of the BCL6 gene (rectangles), with exon 1 at the far right. Exons 1, 2, 3, and parts of exons 4 and 11 are untranslated. The red line in the bottom panel is a magnified view of the 2156-bp break window within BCL6 intron 1. The numbering corresponds to human chromosome 3q (NCBI36/hg 18). The open triangles denote BCL6 breaks in IgH–BCL6 translocations, the gray triangles denote BCL6 breaks in IgL translocations (11 λ and 1 κ), and the filled black triangles denote BCL6 breaks in non-Ig translocations.
First, we noted that the 74 BCL6 breakpoints in Ig–BCL6 translocations show a highly significant proximity to the sequence motif WGCW (P = .004, binomial test; 28% of breaks within 4 nt of WGCW), which is highly enriched in SH regions and targeted for deamination by AID during CSR (supplemental Table 1 and supplemental Figure 4).5 We also found significant proximity to the related sequence motifs WRC and WGC, which are targeted for deamination by AID during SHM in germinal center B cells (supplemental Table 1 and supplemental Figure 3). These proximities are significant by the binomial test, the Student t test, and the Mann-Whitney U-test (P < .005). In contrast, no significant proximity was identified to motifs such as GGC, GTC, GAC, and GCC, which are not efficiently targeted by AID in biochemical assays,6,7 or to the CpG and CGC motifs used in pre-B/pro-B-type translocations (supplemental Tables 1-4 and supplemental Figures 5, 6, 9, and 10).
We recently reported that MYC breakpoints in human Burkitt lymphomas and murine IgH–MYC translocations also occur at WGCW motifs.3 The use of WGCW and other canonical AID motifs at the BCL6, MYC, and Ig loci in IgH–MYC and Ig–BCL6 translocations suggests a common germinal center origin for these rearrangements and strongly implicates AID in their pathogenesis.
Second, in marked contrast to the Ig–BCL6 translocations, the 46 BCL6 breakpoints that partner with non-Ig loci in non-Ig–BCL6 translocations show a highly significant proximity to CpG (P < .003; 15 [33%] of 46 breaks directly at CpG) and to the related motif CGC (P < .01; 12 [26%] of 46 breaks at CGC) (Figure 2A, supplemental Figure 7, and supplemental Table 2). This finding was unexpected, as we previously identified CpG and CGC breakpoints only in IgH translocations occurring at the pro-B/pre-B stage.1,2,8,9 However, sequence analysis of the BCL6 breakpoint junctions strongly suggests that these translocations are not occurring in pro-B/pre-B cells (see supplemental Results and Discussion). In particular, we found no significant evidence of RAG or terminal deoxynucleotidyl transferase activity, which are characteristic of normal V(D)J junctions and pro-B/pre-B translocations such as IgH–BCL2 and IgH–CCND1 (supplemental Table 6). Moreover, 25 (44%) of the 57 breakpoints show evidence of junctional microhomology, a feature that is characteristic of non-homologous DNA end-joining repair but is infrequent in normal V(D)J rearrangements and pre-B/pro-B translocations.
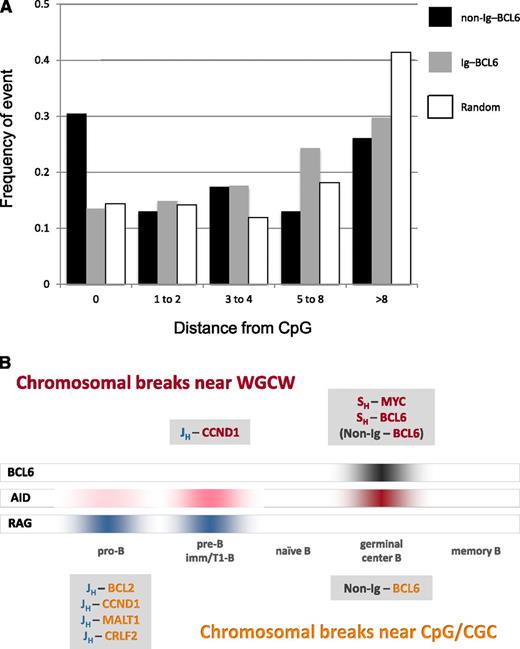
BCL6 breaks occur preferentially at CpG and WGCW motifs. (A) Distance from BCL6 breakpoint to CpG motif in Ig–BCL6 and non-Ig–BCL6 translocations. The proportions of observed BCL6 breakpoints that occur directly at a CpG site (0 bp) and at distances of 1 to 2 bp, 3 to 4 bp, 5 to 8 bp, and greater than 8 bp from the nearest CpG site are shown in black (non-Ig rearrangements) and in gray (Ig translocations). For comparison, the corresponding proportions for a random distribution of breaks in the 2156-bp BCL6 break window are shown in white. The heights of the white bars reflect the density and spacing of CpG motifs in the break region. For example, 32.6% of the BCL6 breaks in non-Ig rearrangements (P < .003; supplemental Table 2), but only 13.5% of the BCL6 breaks in Ig translocations (P > .15; supplemental Table 1), occur directly at CpG compared with 15.3% for a random distribution of breaks in this region. (B) Timing of chromosomal translocations as a function of B-cell development. Breakpoint motif analysis suggests that most lymphoma translocations occur either in germinal center B-cells, when AID and BCL6 are highly expressed, or in pro-B/pre-B cells, when the RAG complex is highly expressed and AID is expressed at very low levels. The Ig breaks in most Ig-MYC and Ig-BCL6 translocations are in SH regions, which contain hundreds of WGCW repeats, and the MYC and BCL6 breaks occur near WGCW and related motifs scattered throughout these partner loci (red text in figure). The Ig breaks in most pro-B/pre-B cell translocations are generated at JH and DH segments by the RAG complex as part of the V(D)J recombination process (blue text). Most CpG breaks occur at Ig partner loci in pro-B/pre-B cell translocations, but also in a subset of non-Ig–BCL6 rearrangements that probably occur in germinal center B-cells (orange text).
BCL6 breaks occur preferentially at CpG and WGCW motifs. (A) Distance from BCL6 breakpoint to CpG motif in Ig–BCL6 and non-Ig–BCL6 translocations. The proportions of observed BCL6 breakpoints that occur directly at a CpG site (0 bp) and at distances of 1 to 2 bp, 3 to 4 bp, 5 to 8 bp, and greater than 8 bp from the nearest CpG site are shown in black (non-Ig rearrangements) and in gray (Ig translocations). For comparison, the corresponding proportions for a random distribution of breaks in the 2156-bp BCL6 break window are shown in white. The heights of the white bars reflect the density and spacing of CpG motifs in the break region. For example, 32.6% of the BCL6 breaks in non-Ig rearrangements (P < .003; supplemental Table 2), but only 13.5% of the BCL6 breaks in Ig translocations (P > .15; supplemental Table 1), occur directly at CpG compared with 15.3% for a random distribution of breaks in this region. (B) Timing of chromosomal translocations as a function of B-cell development. Breakpoint motif analysis suggests that most lymphoma translocations occur either in germinal center B-cells, when AID and BCL6 are highly expressed, or in pro-B/pre-B cells, when the RAG complex is highly expressed and AID is expressed at very low levels. The Ig breaks in most Ig-MYC and Ig-BCL6 translocations are in SH regions, which contain hundreds of WGCW repeats, and the MYC and BCL6 breaks occur near WGCW and related motifs scattered throughout these partner loci (red text in figure). The Ig breaks in most pro-B/pre-B cell translocations are generated at JH and DH segments by the RAG complex as part of the V(D)J recombination process (blue text). Most CpG breaks occur at Ig partner loci in pro-B/pre-B cell translocations, but also in a subset of non-Ig–BCL6 rearrangements that probably occur in germinal center B-cells (orange text).
The BCL6 breaks in these non-Ig translocations also show proximity to WGCW (P < .05 for binomial, Student t, and Mann-Whitney U tests), WRC (P < .04, binomial and Mann-Whitney U), and WGC (P = .03, Mann-Whitney U) motifs, suggesting that these breaks also are initiated by AID. The statistical significance of these motifs appears to be weaker for non-Ig–BCL6 than Ig–BCL6 translocations (supplemental Tables 1 and 2), but this difference might reflect the fact that fewer non-Ig breaks were available for analysis and that a third of the non-Ig breaks occur at CpG/CGC.
Our findings strongly implicate AID in the pathogenesis of BCL6 translocations. Previous studies have shown that AID-dependent SHM causes mutations at BCL6.10-13 Indeed, the distribution of BCL6 mutations is virtually identical to that of BCL6 translocation breakpoints (supplemental Figure 13). Aberrant SHM also causes mutations, to a lesser extent, at other genes actively transcribed in germinal center B-cells, including the BCL6 translocation partners PIM1, RHOH, POU2AF1, and CTIIA.14-16 Other studies have shown that SHM causes not only mutations but also double-strand breaks.17,18 In light of these studies, our results strongly suggest that BCL6 translocations are initiated by AID in germinal center B-cells (Figure 2B).
The basis for the highly focused clustering of BCL6 breakpoints and SHM mutations in the same 2156-bp region near the beginning of BCL6 intron 1 is unclear (supplemental Figure 13). This is likely a consequence of several factors that are yet to be defined, such as chromatin accessibility to AID. In this regard, it is important to note that the upstream portion of BCL6 intron 1 has a favorable H3K4me3 histone pattern for AID accessibility (Figure 1 and supplemental Figures 11 and 12).16,19,20
It also is unclear why BCL6 breakpoints occur at CpG/CGC motifs in non-Ig–BCL6 rearrangements (but not in Ig–BCL6 translocations), particularly because both rearrangement types appear to be initiated by AID in germinal center B-cells. One possibility is that differences in the kinetics of chromosomal breakage at Ig and non-Ig loci might somehow influence which sequence motif is used at BCL6. For example, AID is much less active at non-Ig than at Ig loci,14,15 so the generation of non-Ig breaks is more likely to be rate-limiting than Ig breaks, which are efficiently introduced by CSR and SHM. In principle, the limited availability of non-Ig breaks should favor the selection of BCL6 breaks at methylated CpG/CGC motifs, where AID causes long-lived T:G mismatches (in comparison with short-lived U:G mismatches at nonmethylated WGCW/WRC/WGC sites).2 Alternatively, CSR-associated breaks at IgH (but not non-Ig breaks) might preferentially induce breaks at BCL6 by recruiting and activating the Artemis DNA nuclease.21 A third possibility is that the level of AID, which varies widely in different germinal center B-cell subsets,22 determines the preferred motif.
In summary, we identify the first breakpoint sequence motifs in BCL6 translocations, and we show that these motifs are differentially used in Ig–BCL6 and non-Ig–BCL6 rearrangements. Our findings support previous analyses demonstrating potential biological and clinical differences between diffuse large B-cell lymphoma cases having Ig–BCL6 and non-Ig–BCL6 rearrangements.23,24
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The work was supported in part by the National Institutes of Health (M.R.L.).
Authorship
Contribution: Z.L., A.G.T., H.A.G., and M.R.L. designed the research; T.A. and H.O. cloned and sequenced 66 of the 137 breakpoints in this study (supplemental Table 8); Z.L., A.G.T., H.A.G., and M.R.L. analyzed and interpreted the data; Z.L. and A.G.T. performed statistical analyses; Y.J. and A.M.M. did the genome-wide analysis of histone marks; and Z.L., H.A.G., and M.R.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.G.T. is Department of Pathology, Stanford University, Stanford, CA. The current affiliation for T.A. and H.O. is Department of Hematology, Tenri Hospital, Tenri, Nara, Japan.
Correspondence: Harvey A. Greisman, Department of Laboratory Medicine, University of Washington Medical Center, Box 357110, Seattle, WA 98195; e-mail: greisman@uw.edu.
References
Author notes
Z.L. and A.G.T. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal